Welcome to China Oncology,
China Oncology ›› 2025, Vol. 35 ›› Issue (6): 605-630.doi: 10.19401/j.cnki.1007-3639.2025.06.011
• Guideline and Consensus • Previous Articles
China Anti-Cancer Association Committee of Rehabilitation and Palliative Care, Fujian Anti-Cancer Association Committee of Cancer Pain
Received:2025-03-10
Revised:2025-03-27
Online:2025-06-30
Published:2025-07-14
Supported by:Share article
CLC Number:
China Anti-Cancer Association Committee of Rehabilitation and Palliative Care, Fujian Anti-Cancer Association Committee of Cancer Pain. Chinese expert consensus on whole-process management of chemotherapy-related diarrhea (2025 edition)[J]. China Oncology, 2025, 35(6): 605-630.
Tab. 1
Evidence quality and recommendation strength grading in the GRADE system"
| 证据级别及推荐强度分级 | 具体描述 | 研究类型 |
|---|---|---|
| 证据质量分级 | ||
| 高质量证据 | 非常确信真实的效应值接近效应估计值 | ① 随机对照研究; ② 质量升高二级的观察性研究 |
| 中质量证据 | 对效应估计值有中等程度的信心:真实值有可能接近估计值,但仍存在二者大不相同的可能性 | ① 质量降低一级的随机对照研究; ② 质量升高一级的观察性研究 |
| 低质量证据 | 对效应估计值的确信程度有限:真实值可能与估计值大不相同 | ① 质量降低二级的随机对照研究; ② 观察性研究 |
| 极低质量证据 | 对效应估计值几乎没有信心:真实值很可能与估计值大不相同 | ① 质量降低三级的随机对照研究; ② 质量降低一级的观察性研究; ③ 系列病例观察; ④ 个案报道 |
| 推荐强度分级 | ||
| 强推荐 | 遵从推荐时利大于弊 | |
| 弱推荐 | 遵从推荐时利弊不确定或利弊相当 |
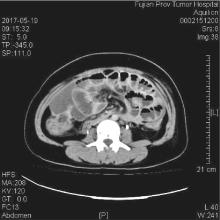
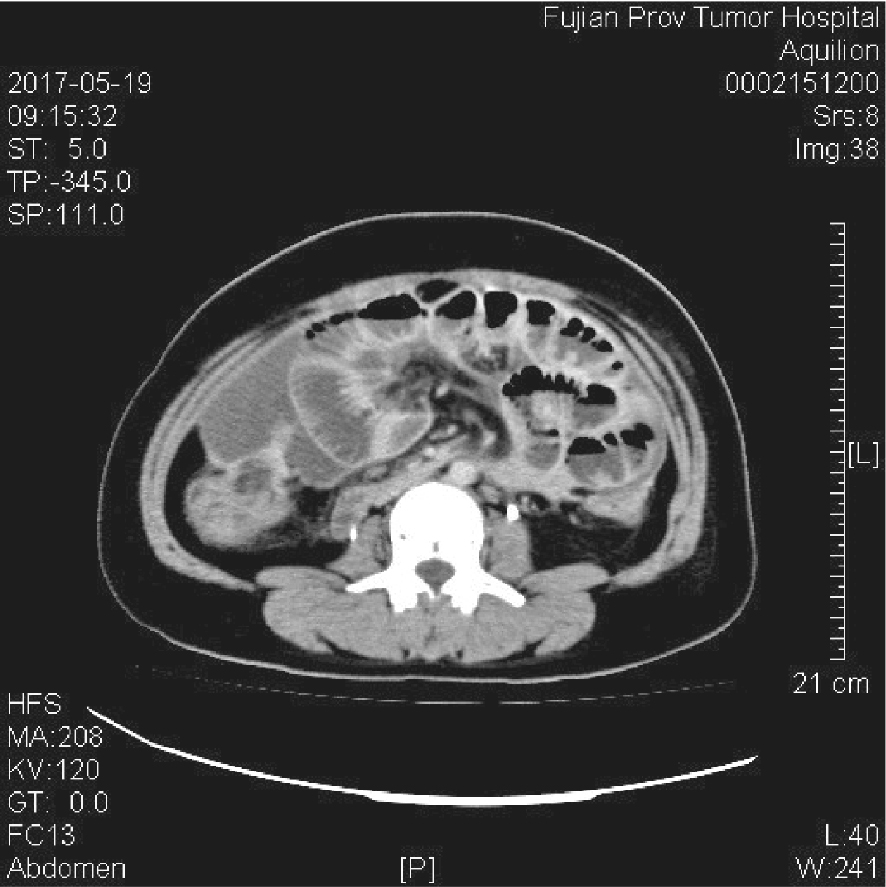
Fig. 2
A 46-year-old female with gastric signet-ring cell carcinoma and peritoneal metastasis developed neutropenic enterocolitis following chemotherapy with paclitaxel, oxaliplatin, and 5-fluorouracil/leucovorin (POF regimen) CT findings demonstrated diffuse intestinal dilatation and bowel wall thickening (>4 mm) throughout the abdominal cavity, with particularly asymmetric thickening of the cecum (Fujian Cancer Hospital, May 19, 2017)."
Tab. 3
Overview of diarrhea incidence induced by different chemotherapeutic agents[1]"
| 药物类别 | 常见化疗药物 | 严重程度(1~4级) |
|---|---|---|
| 拓扑异构酶抑制剂 | 伊立替康 | 所有级别:50%~88% 3~4级:3%~22% |
| 拓扑替康 | ||
| 伊立替康脂质体 | ||
| 依托泊苷 | ||
| 替尼泊甙 | ||
| 抗代谢药物 | 5-氟尿嘧啶 | 所有级别:80% 3~4级:54% |
| 卡培他滨 | ||
| 替吉奥 | ||
| 甲氨蝶呤 | ||
| 烷化剂 | 环磷酰胺 | 所有级别:20% |
| 异环磷酰胺 | ||
| 美法仑 | ||
| 白消安 | ||
| 卡莫司汀和洛莫司汀 | ||
| 达卡巴嗪 | ||
| 苯丁酸氮芥 | ||
| 美法仑 | ||
| 氮芥 | ||
| 有丝分裂抑制剂 | 紫杉醇 | 1~2级:50% 3~4级:3%~5% |
| 多西他赛 | ||
| 白蛋白结合型紫杉醇 | ||
| 长春花碱 | ||
| 长春新碱 | ||
| 铂类药物 | 顺铂 | 1~2级:70%~80% 3~4级:2.6% |
| 卡铂 | ||
| 奥沙利铂 | ||
| 抗叶酸药物 | 培美曲塞 | 1~2级:7%~13% |
| 蛋白酶体抑制剂 | 硼替佐米 | 所有级别:51% 3~4级:8% |
| 卡非佐米 | ||
| 伊沙佐米 | ||
| 组蛋白去乙酰化酶抑制剂 | 伏立诺他 | 所有级别:23%~52% 3~4级:2% |
| 贝利诺他 | ||
| 免疫调节酰亚胺药物 | 来那度胺 | 所有级别:6%~22% 3~4级:1% |
| 泊马度胺 |
Tab. 4
Dietary recommendations during diarrhea[86]"
| 食物组 | 耐受性较好的食物 | 应限制或避免的食物 |
|---|---|---|
| 乳制品 | 酪乳 脱脂、低脂和淡奶 豆奶/杏仁奶/米奶/椰奶 酸奶 低脂、部分脱脂和陈年奶酪 | 全脂牛奶 半乳 奶油 酸奶油 普通(全脂)冰淇淋 加浆果、干果或坚果的酸奶 大多数奶酪 提示:如果您发现自己对乳制品敏感,请尽量避免食用乳糖。选择不含乳糖的牛奶、酸奶、奶酪等 |
| 谷物 | 用白面粉或精制面粉制成的面包、面条及相关食品 白米 小麦精、米糊 用精制谷物制成,不添加纤维的谷物食品(膨化米、玉米片) | 全麦或全麦制品 糙米或野生稻 大麦、燕麦和其他全谷物 用全麦或麸皮制成的谷物 用种子或坚果制成的固体食品 爆米花 提示:选择每份膳食纤维含量少于2 g的谷物食品 |
| 水果/蔬菜 | 无果肉果汁 熟香蕉 瓜类 去皮的新鲜苹果、梨或桃子等水果 罐装软水果 大多数煮熟的蔬菜(没有种子或皮) 生菜(如果嫩且去筋) 过滤的蔬菜汁 无皮土豆 | 大多数生水果 干果 带果肉的果汁 浓糖浆罐装水果 任何加山梨醇的水果 梅干汁 大多数生蔬菜 油炸蔬菜 甜菜、西兰花、球芽甘蓝、卷心菜、 花椰菜 羽衣甘蓝、芥菜和芜菁叶等粗纤维蔬菜 整颗玉米粒 土豆和红薯皮 |
| 蛋白质 | 嫩而熟透的肉、猪肉、家禽、鱼、蛋或不添加脂肪的大豆食品 滑腻的坚果酱(如果可以耐受) | 煎炸的肉、家禽或鱼 加工肉类 肥肉和软骨肉 整颗坚果、大块坚果酱 |
| 饮料 | 水 肉汤 脱咖啡因咖啡 不含咖啡因的茶 稀释果汁或果汁饮料 口服补液饮料 | 含咖啡因的饮料,包括普通咖啡、普通茶、可乐和能量饮料 含有高果糖玉米糖浆或山梨醇的饮料 酒精饮料 提示:您可能每日比健康人需要喝更多水来补充因腹泻而流失的水分。 |
| 脂肪/其他 | 脂肪包括油、黄油、奶油、奶油干酪、人造黄油、蛋黄酱和沙拉酱 提示:每天限制脂肪摄入量 | 糖醇,如木糖醇和山梨醇 蜂蜜 |
Tab. 5
Recommended Traditional Chinese Medicine (TCM) formulations for chemotherapy-induced diarrhea based on syndrome differentiation"
| 证型 | 方剂 | 治法 | 成分 |
|---|---|---|---|
| 寒热错杂 | 半夏泻心汤 | 调和寒热,散结消痞 | 干姜6 g、人参15 g、黄芩6 g、黄连6 g、半夏15 g、甘草6 g、大枣10 g |
| 湿热下注 | 黄芩汤 | 清热解湿,止泻 | 黄芩9 g、白芍9 g、甘草6 g、大枣10 g |
| 葛根芩连汤 | 解表散邪,清热止泻 | 葛根10 g、黄芩6 g、黄连3 g、甘草6 g | |
| 脾虚湿盛 | 参苓白术散 | 健脾益气,化湿止泻 | 人参15 g、茯苓15 g、白扁豆30 g、白术15 g、甘草6 g、桔梗6 g、莲子肉9 g、山药15 g、薏苡仁30 g、砂仁6 g |
| 肝郁脾虚 | 痛泻要方 | 疏肝健脾,缓痛止泻 | 白术18 g、白芍12 g、陈皮9 g、防风6 g |
| 水饮内停 | 五苓散 | 温阳化气,化湿止泻 | 泽泻15 g、猪苓9 g、桂枝6 g、白术9 g |
| 脾虚气陷 | 补中益气汤/丸 | 补中健脾,升阳止泻 | 黄芪15 g、人参15 g、炙甘草6 g、炒白术10 g、陈皮6 g、当归10 g、升麻6 g、柴胡12 g、生姜6 g、大枣10 g |
| 肾虚泄泻 | 四神丸 | 温肾暖脾,涩肠止泻 | 肉豆蔻6 g、五味子6 g、补骨脂10 g、吴茱萸10 g |
| 外感风寒,内伤湿滞 | 藿香正气散 | 散寒化湿,和中止泻 | 大腹皮12 g、白芷6 g、紫苏6 g、茯苓12 g、半夏曲9 g、白术9 g、陈皮6 g、厚朴9 g、苦桔梗6 g、藿香9 g、甘草6 g、生姜6 g、大枣10 g |
Tab. 6
Summary of clinical issues and recommendations for the comprehensive management of chemotherapy-induced diarrhea"
| 临床问题 | 推荐意见(专家共识) | 推荐强度 |
|---|---|---|
| 临床问题1:化疗相关腹泻的定义 | 根据世界卫生组织的相关意见和美国国家癌症研究所常见不良反应术语标准(National Cancer Institute-common terminology criteria for adverse events,NCI-CTCAE 5.0),本专家组将化疗相关腹泻定义为:每日排出3次或以上稀便或水样便(或排便频率高于个人正常频率)。频繁排成型大便不应被视为腹泻。对于造口患者,造口排出物为稀便或水样便,并且较基线水平有所增加,也应视为腹泻 | 弱推荐 |
| 临床问题2:认识中性粒细胞减少性小肠结肠炎的意义 | 在采用高强度化疗方案的患者群体中,中性粒细胞减少性小肠结肠炎的发生率并不低。由于化疗后患者肠黏膜通透性增加,同时在化疗引发中性粒细胞减少的情形下,患者易罹患中性粒细胞减少性小肠结肠炎。这是一个由轻到重的连续过程,大多数患者在早期仅表现为单纯腹泻,且通常可自愈。对于小部分未能自愈的患者,如果没有进行及时干预,病情可以逐渐加重,最终发展为麻痹性肠梗阻、腹膜炎,甚至可能出现肠穿孔,最终导致死亡 | 弱推荐 |
| 临床问题3:化疗相关腹泻的分级 | NCI-CTCAE 5.0分级是目前最常用的腹泻分级系统。尽管这一分级系统在临床应用广泛,但也存在一些局限性。它未涵盖腹泻的量和持续时间,未考虑腹部绞痛等主观不适,未考虑患者对症状严重程度的主观感知。因此,目前临床上迫切需要更加全面的腹泻评估方法 | 弱推荐 |
| 临床问题4:复杂性腹泻的定义 | 根据美国临床肿瘤学会指南,复杂性腹泻的定义如下:NCI-CTCAE 5.0版分级为3/4级腹泻,或1/2级腹泻伴有以下任何1种风险因素,包括中重度腹痛、恶心/呕吐(≥2级)、体能状态下降、发热、脓毒血症、中性粒细胞减少、明显出血和脱水。相反,仅有NCI-CTCAE 5.0版分级为1/2级腹泻且无上述风险因素的患者被归类为非复杂性腹泻 | 强推荐 |
| 临床问题5:化疗相关腹泻患者的相关实验室检查 | 实验室检查应根据患者的临床状况(病情越重检查越为必要)、症状持续时间以及是否存在明确病因来选择相应的检查。对于体能状态良好且病因明确的患者[如刚接受5-氟尿嘧啶和(或)伊立替康治疗],通常无需过多检查。而对于复杂性腹泻患者,应进行血液全血细胞计数、肝肾功能、电解质、葡萄糖、甲状腺功能、红细胞沉降率、C反应蛋白和降钙素原检测,并进行粪潜血试验、粪培养、粪显微镜检查和艰难梭菌检测。但粪培养的阳性诊断率通常<5%。有条件的医疗中心还可以检测粪钙卫蛋白和乳铁蛋白。如果患者发热,还应进行血培养。如果患者血压过低或心动过速,建议检测血液的酸碱度和乳酸浓度。其他检查内容还包括血清淀粉酶浓度或粪便胰腺弹性蛋白酶-1,以排除胰腺功能不全,尤其应关注曾经接受过腹部放疗、胰腺手术或有过量饮酒史的患者。葡萄糖氢甲烷呼气试验可用于排除小肠细菌过度生长,必要时可考虑经验性抗生素治疗和无乳糖饮食试验 | 强推荐 |
| 临床问题6:化疗相关腹泻的微生物检查 | 粪便样本应包括细菌、真菌和原虫等微生物学检测。必要时,还应考虑志贺氏菌、沙门氏菌、耶尔森菌、弯曲杆菌、产志贺毒素大肠杆菌O157:H7等菌株,以及贾第鞭毛虫、隐孢子虫、溶组织内阿米巴、微孢子虫/环孢子虫等孢子虫属、人囊芽原虫和人滴虫等。如果患者有反复灌肠、长时间放置鼻胃管、胃肠道手术史或使用抗生素史,应考虑艰难梭菌感染的可能。艰难梭菌检测,粪便样本可先进行核酸扩增试验或谷氨酸脱氢酶试验,阳性患者再进行艰难梭菌毒素A和B的检测确诊。然而,一些患者在化疗后未使用过任何抗生素,仍可能发生艰难梭菌结肠炎,这可能与化疗引起的肠道损伤促进艰难梭菌的增殖有关。 如果十二指肠镜和结肠镜检查发现小溃疡或糜烂,应进行活检以排除病毒感染(尤其是巨细胞病毒感染)。如果溃疡严重,可开始经验性抗巨细胞病毒治疗(如:更昔洛韦)。十二指肠镜抽吸物可用来排除小肠细菌过度生长和寄生虫感染。对于血性腹泻患者,应至少检测肠出血性大肠杆菌和溶组织内阿米巴。血性粪便还应确定是否含有志贺毒素(确定是否为O157:H7或非O157产志贺毒素大肠杆菌菌株),并检测粪便中的白细胞或乳铁蛋白。如果粪便白细胞/乳铁蛋白检测结果为阴性,还应进一步检测肠阿米巴[ | 弱推荐 |
| 临床问题7:CT在中性粒细胞减少性小肠结肠炎中的诊断价值 | 如果怀疑中性粒细胞减少性小肠结肠炎,应进行CT扫描。CT是诊断此病的首选影像学手段,能有效评估肠管扩张和肠壁增厚,尤其能够发现盲肠的明显不对称增厚。CT检查结果与中性粒细胞减少性小肠结肠炎的临床预后存在一定关系,肠壁厚度>10 mm的患者死亡率较高 | 弱推荐 |
| 临床问题8:化疗相关腹泻患者的内镜检查 | 化疗相关腹泻通常没有内镜检查指征。然而,在一些情况下,内镜检测有助于排除其他潜在的病因。例如,十二指肠镜和结肠镜可用于排除病毒感染,十二指肠镜还可用于排除小肠细菌过度生长和寄生虫感染。中性粒细胞减少或肠黏膜脆弱的患者应避免内镜检查,因为肠穿孔的风险增加。此外,在中性粒细胞减少的患者中,由于免疫抑制,典型的假膜可能无法形成。在某些情况下,内镜还可以用于评估肠道损伤的严重程度,从而指导治疗决策 | 强推荐 |
| 临床问题9:药物基因组学在5-氟尿嘧啶相关腹泻的预测作用 | DPYD的基因多态性可预测约20%的5-氟尿嘧啶相关腹泻。胸苷酸合酶基因多态性也可能与5-氟尿嘧啶相关毒性风险增加相关,但与DPYD基因多态性相比,相关数据尚不确定。其他潜在的预测标志物的作用仍需进一步研究。 所有计划接受氟嘧啶类药物治疗的患者都应进行相关咨询,让患者了解二氢嘧啶脱氢酶缺乏可能导致的严重甚至危及生命的不良反应。可以考虑与患者讨论是否进行DPYD基因型的预先检测。然而,胸苷酸合酶基因的预先检测并不推荐。在初次接受氟嘧啶类药物就出现严重或意外毒性反应的患者,均应接受DPYD基因型检测。如果条件许可,也可同时进行胸苷酸合酶基因型检测 | 弱推荐 |
| 临床问题10:出现DPYD基因多态性变异,5-氟尿嘧啶剂量的调整策略 | 本专家组建议所有存在DPYD基因多态性且酶活性评分为1或1.5(DPYD多态性变异对应酶活性评分可以参考临床药物基因组学实施联盟制定的二氢嘧啶脱氢酶基因型和5-氟尿嘧啶给药指南)的患者均应将5-氟尿嘧啶的标准剂量减少50%,然后再根据患者毒性反应决定增加剂量或继续减量,甚至停用5-氟尿嘧啶。如果有条件,还可以结合治疗药物监测。对于二氢嘧啶脱氢酶缺乏的患者,喹唑啉叶酸类似物雷替曲塞作为胸苷酸合酶抑制剂,可考虑作为5-氟尿嘧啶的有效替代药物。对于已经出现毒性反应的患者,如果肾功能正常,则无需进行透析,因为即使二氢嘧啶脱氢酶完全缺乏,5-氟尿嘧啶也能通过尿液迅速清除 | 强推荐 |
| 临床问题11:接受伊立替康治疗患者的UGT1A1基因多态性检测 | 初始使用伊立替康患者,是否进行预先检测存在一定争议。本专家组建议有条件的医疗机构在使用伊立替康前,进行UGT1A1基因多态性检测。如果发现高风险基因型,建议将伊立替康初始剂量降低50%。之后可根据患者的毒性反应进一步调整剂量。如果临床怀疑患有吉尔伯特综合征(无胆红素尿和明显溶血但出现高间接胆红素血症)的患者,应进行UGT1A1基因型检测 | 弱推荐 |
| 临床问题12:多药联合化疗方案与化疗相关腹泻 | 以5-氟尿嘧啶、伊立替康和奥沙利铂联合化疗方案为代表的多药联合化疗方案,在呈现卓越疗效的同时,也带来更多更严重的毒性反应。更强烈的化疗方案可能导致更严重的肠道黏膜损伤、肠道菌群失衡、中性粒细胞减少以及全身免疫抑制。近年来,随着多药联合化疗方案应用的增加,化疗相关腹泻的发生率和相关死亡率也明显上升。因此,临床上应更加重视多药联合化疗方案相关腹泻的处理,尤其要警惕中性粒细胞减少性小肠结肠炎的发生 | 强推荐 |
| 临床问题13:新型封装制剂伊立替康脂质体是否可以改善化疗相关腹泻的发生率? | 伊立替康脂质体是一种纳米脂质体封装制剂。研究表明,伊立替康脂质体相关腹泻发生率可能低于普通伊立替康,而且在亚洲人群的腹泻发生率也低于全球数据。伊立替康脂质体是否可以在中国人群中降低腹泻的发生率值得进一步探索。UGT1A1*28的纯合突变未见对伊立替康脂质体毒性产生影响,但是在亚洲人群更常见的UGT1A1*6基因多态性是否影响伊立替康脂质体相关腹泻仍需进一步研究 | 强推荐 |
| 临床问题14:腹泻患者的饮食建议 | 腹泻患者应选择易消化的清淡食物,避免辛辣刺激性食物和高渗透性膳食补充剂,避免酒精和咖啡因等刺激性物质。对于乳糖不耐受的患者,应适当限制乳糖摄入。根据胃肠道耐受情况适当限制富含膳食纤维的生蔬菜及新鲜水果 | 强推荐 |
| 临床问题15:腹泻患者的口服补液原则 | 口服补液治疗适用于1/2级腹泻患者。对于老年患者以及2级腹泻患者更提倡口服补液盐(oral rehydration salts,ORS)。对于老年患者,尤其合并慢性心脏或肾脏功能衰竭的患者,应避免过度补液。但补液量应高于尿量加上估计的不显性丢失(通常为30~50 mL/h)和胃肠道丢失量 | 弱推荐 |
| 临床问题16:化疗相关腹泻患者的静脉补液原则 | 对于3/4级腹泻或出现严重脱水迹象的患者,静脉补液应作为首选治疗。如果对于低血容量情况不明的患者,首选500 mL平衡盐溶液(如果钾浓度高于5.5 mmol/L或怀疑少尿性急性肾损伤,应选用0.9%的生理盐水)进行快速补液。必要时可补充白蛋白保持血浆胶体渗透压,并评估患者对输液的反应。应避免使用低渗性液体进行静脉补液。如果初始使用0.9%的生理盐水进行补液,待确认血钾浓度并达到正常的尿量后,应改为平衡盐溶液(如林格氏乳酸盐和醋酸盐溶液),以降低诱发高氯性酸中毒的风险,但呕吐等原因导致低氯性酸中毒的患者除外。对于合并低血压、心动过速或可能存在脓毒血症且乳酸浓度高的重症患者,应给予30 mL/kg的初始液体输注。对于低钾血症患者,如果尿量>0.5 mL/(kg·h-1),应同步补钾。应监测中心静脉压和尿量,液体平衡的目标应该是保持足够的中心静脉压(8~12 mmHg)和尿量[>0.5 mL/(kg·h-1)],且乳酸浓度不升高。此时可调整液体复苏速度,以避免液体超负荷。若中心静脉压显示尽管已经充分扩容但仍存在少尿性急性肾损伤[<0.5 mL/(kg·h-1)],则必须紧急寻求重症监护专家或肾病专家会诊,因为存在发生肺水肿的风险。若患者对治疗反应良好,应持续补液直至血容量及循环状态恢复稳定,之后转为适当的治疗方案以补充持续的液体流失 | 强推荐 |
| 临床问题17:化疗相关腹泻患者的营养和支持建议 | 对于腹泻影响到进食量的患者,特别是接受强烈化疗的患者,应根据患者的能量摄入量提供相应的外周营养支持。建议的总体能量摄入应在25~30千卡/(kg·d-1),蛋白质摄入量1.0~1.5 g/ (kg·d-1)。同时,应密切关注全血细胞变化趋势,及时给予治疗性或预防性升白细胞治疗,以降低合并肠道感染风险 | 强推荐 |
| 临床问题18:抗生素在化疗相关腹泻中的应用 | 随着FOLFOXIRI或FOLFIRINOX等多药联合化疗方案的推广,化疗相关腹泻和中性粒细胞减少症的发生率明显上升,临床出现了较多因腹泻导致患者死亡的情况。专家组认为,这种现象类似于IFL方案的情况,患者从早期致病菌在消化道定植并逐渐恶化,最终发展为中性粒细胞减少性小肠结肠炎,这是导致死亡的关键因素。专家组建议,对于洛哌丁胺治疗无效的腹泻患者,应早期开始口服广谱抗生素(包括抗真菌药物)进行经验性治疗,以阻止向小肠结肠炎的发展。如果口服抗生素治疗无效,可考虑改为静脉使用强效广谱抗生素,抗生素应覆盖肠道的革兰氏阴性菌、革兰氏阳性菌和厌氧菌。推荐的初始经验性抗生素包括哌拉西林-他唑巴坦、亚胺培南-西司他丁,或头孢吡肟/头孢他啶与甲硝唑联合治疗。此外,抗真菌药物也应常规使用,因为真菌感染在这些患者中较为常见,并且真菌感染的症状常不典型,粪便检出率低。在抗真菌药物的选择上,推荐使用氟康唑、伏立康唑等广谱抗真菌药物。此外,应依据病原学检测结果进行个体化治疗。如果不能立即排除假膜性结肠炎,可考虑使用甲硝唑;若怀疑有巨细胞病毒感染,可考虑进行抗病毒治疗。对于微生物学检测阳性的患者,应根据检测结果及时调整抗生素使用策略 | 强推荐 |
| 临床问题19:中医中药在化疗相关腹泻的应用 | 虽然现有数据并不完全一致,但本专家组认为中药可以减少或减轻化疗导致的腹泻。中医注重辩证施治,目前临床有多种治疗腹泻有效的中药方剂,应根据患者中医证型和体质选用不同方剂的治疗腹泻。 | 弱推荐 |
| 临床问题20:非复杂性腹泻处理流程 | 对于发生非复杂性腹泻患者,应停止包括口服在内的所有化疗。由于无需快速液体复苏,最初患者可以在家中进行保守治疗,包括口服补液和饮食调整(如限制乳糖摄入和避免高渗透性膳食补充剂)。当1级腹泻持续超过24 h或出现2级腹泻时,应使用洛哌丁胺进行治疗。洛哌丁胺初始剂量为4 mg,然后每4 h 2 mg,或每次排便稀便后服用2 mg,日最高剂量不超过16 mg。腹泻缓解后,应继续服用洛哌丁胺12 h。但洛哌丁胺总服用时长应不超过48 h。如果腹泻缓解,应指导患者在原有改变饮食的基础上,逐渐添加固体食物,并逐渐过渡到正常饮食。如果服用洛哌丁胺期间腹泻持续超过24 h,可加服或改用可待因(30~60 mg/次,每日4次,日最高剂量不超过240 mg)或奥曲肽(100~150 μg/次,皮下注射,每日3次,腹泻停止后24 h停用)。如果腹泻仍持续,建议粪便微生物检测,同时改用或联合口服广谱抗生素(包括抗真菌治疗)进行经验性治疗 | 强推荐 |
| 临床问题21:伊立替康早发型腹泻及药物处理 | 伊立替康早发型腹泻是在用药期间或用药后24 h内发生。这是急性胆碱能反应的一部分,通常伴有腹痛、结膜炎、鼻炎、低血压、血管舒张、出汗、寒战、全身不适、头晕、视力模糊、瞳孔缩小、流泪,流涎增多等症状。 阿托品0.3~0.5 mg皮下注射可用于控制这些症状,必要时阿托品可重复注射。阿托品也可用在伊立替康给药前来预防以上症状的出现。早发型腹泻不应使用止泻药物(如洛哌丁胺) | 强推荐 |
| 临床问题22:伊立替康迟发性腹泻的药物处理 | 迟发型腹泻是在注射伊立替康24 h后出现。处理原则同上述非复杂性腹泻处理流程相同。但在患者第1次出现稀便时,就应开始大量饮用含电解质饮料,并立即开始服用高剂量洛哌丁胺(初始剂量为4 mg,然后每2 h服用2 mg,持续到最后1次稀便后12 h)。由于存在麻痹性肠梗阻的风险,洛哌丁胺的服用时间不得超过48 h | 强推荐 |
| 临床问题23:复杂性腹泻处理流程 | 复杂性腹泻可危及生命,需要积极治疗。患者应进行全血细胞计数、肝肾功能、电解质、葡萄糖、甲状腺功能、红细胞沉降率、C反应蛋白和降钙素原等实验室检查。大多数此类患者应住院进行静脉输液、药物止泻治疗(如洛哌丁胺、奥曲肽等)、心血管状态监测、全血细胞、肾功能、电解质和体液出量的连续评估,并经静脉使用广谱抗生素(包括抗真菌治疗)进行经验性治疗。对于3级腹泻且尚未经洛哌丁胺充分治疗或口服广谱抗生素治疗,水分和能量摄入充足,没有令人担忧的体征或症状的患者,可先在家中治疗。 如果患者在含5-氟尿嘧啶或伊立替康化疗的第1个周期就出现严重症状(如腹泻、黏膜损伤、骨髓抑制等),应进行DPYD、TMYS或UGT1A1基因多态性检测。但即使检测结果为阴性,也应考虑对药物进行相应的减量或使用替代药物 | 强推荐 |
| 临床问题24:微生物学检测和抗生素使用流程 | 对于尚未进行微生物学检测的患者,应进行粪便微生物学检查。必要时进行内镜排除小肠细菌过度生长、病毒和寄生虫感染。 对于发热、腹膜炎体征或血性腹泻的患者,应紧急进行腹部和盆腔CT扫描,建议血培养,同时进行外科会诊。静脉强效广谱抗生素的使用至关重要。如果不能立即排除假膜性结肠炎,可考虑经验性使用甲硝唑。进一步抗生素调整可根据微生物学检测结果进行。对于内镜发现肠道严重溃疡且怀疑或证实巨细胞病毒感染的患者,可使用抗病毒治疗(如更昔洛韦)。 | 弱推荐 |
| 临床问题25:中性粒细胞减少性小肠结肠炎处理流程 | 如果影像学检查发现患者肠管广泛扩张,肠壁广泛增厚,盲肠壁不对称增厚,提示患者可能存在严重的中性粒细胞减少性小肠结肠炎,特别是在中性粒细胞减少患者中。对难治性腹泻患者,及早经验性使用口服广谱抗生素是降低中性粒细胞减少性小肠结肠炎发生、发展的有效措施。对于诊断为中性粒细胞减少性小肠结肠炎的患者,应及时静脉经验性使用强效广谱抗生素(如哌拉西林-他唑巴坦、亚胺培南-西司他丁,或头孢吡肟/头孢他啶联合甲硝唑治疗,抗真菌治疗也同时使用)。白细胞低下患者应积极进行升白细胞治疗(人粒细胞集落刺激因子)和营养支持治疗。出现肠管广泛扩张患者,可以进行胃肠降压缓解肠道压力,但应避免手术治疗,以及避免使用抗胆碱能药物、止泻药和阿片类药物,因为这些药物可加重肠梗阻 | 强推荐 |
| 临床问题26:化疗相关腹泻的预防措施 | 应预先告知患者某些化疗方案可导致腹泻的风险,并指导患者正确服用洛哌丁胺。建立有效的医患沟通机制是降低化疗相关腹泻发生率及严重程度的关键措施。 奥曲肽作为二级预防的作用仍未得到证实,因此专家组并不推荐奥曲肽作为二级预防措施。口服益生菌作为预防化疗相关腹泻的干预措施,总体益处仍需进一步评估,特别需要警惕在化疗后免疫功能低下的患者中出现更严重的感染风险 | 弱推荐 |
| [1] |
AKBARALI H I, MUCHHALA K H, JESSUP D K, et al. Chemotherapy induced gastrointestinal toxicities[J]. Adv Cancer Res, 2022, 155: 131-166.
doi: 10.1016/bs.acr.2022.02.007 pmid: 35779873 |
| [2] |
MCQUADE R M, STOJANOVSKA V, ABALO R, et al. Chemotherapy-induced constipation and diarrhea: pathophysiology, current and emerging treatments[J]. Front Pharmacol, 2016, 7: 414.
pmid: 27857691 |
| [3] | World Health Organization. Diarrhoeal disease. [EB/OL]. [2024-10-15] https://www.who.int/en/news-room/fact-sheets/detail/diarrhoeal-disease. |
| [4] | National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0[Z]. 2017. |
| [5] |
FATA F, RON I G, KEMENY N, et al. 5-fluorouracil-induced small bowel toxicity in patients with colorectal carcinoma[J]. Cancer, 1999, 86(7): 1129-1134.
pmid: 10506695 |
| [6] | IKUNO N, SODA H, WATANABE M, et al. Irinotecan (CPT-11) and characteristic mucosal changes in the mouse ileum and cecum[J]. J Natl Cancer Inst, 1995, 87(24): 1876-1883. |
| [7] |
STRINGER A M, GIBSON R J, LOGAN R M, et al. Faecal microflora and beta-glucuronidase expression are altered in an irinotecan-induced diarrhea model in rats[J]. Cancer Biol Ther, 2008, 7(12): 1919-1925.
pmid: 18927500 |
| [8] |
PARNES H L, FUNG E, SCHIFFER C A. Chemotherapy-induced lactose intolerance in adults[J]. Cancer, 1994, 74(5): 1629-1633.
pmid: 8062196 |
| [9] | HOLMA R, LAATIKAINEN R, ORELL H, et al. Consumption of lactose, other FODMAPs and diarrhoea during adjuvant 5-fluorouracil chemotherapy for colorectal cancer[J]. Nutrients, 2020, 12(2): 407. |
| [10] |
MCQUADE R M, STOJANOVSKA V, DONALD E L, et al. Irinotecan-induced gastrointestinal dysfunction is associated with enteric neuropathy, but increased numbers of cholinergic myenteric neurons[J]. Front Physiol, 2017, 8: 391.
doi: 10.3389/fphys.2017.00391 pmid: 28642718 |
| [11] |
NYHLÉN A, LJUNGBERG B, NILSSON-EHLE I, et al. Impact of combinations of antineoplastic drugs on intestinal microflora in 9 patients with leukaemia[J]. Scand J Infect Dis, 2002, 34(1): 17-21.
pmid: 11874159 |
| [12] | CHIA D K A, SUNDAR R, KIM G, et al. Outcomes of a phase Ⅱ study of intraperitoneal paclitaxel plus systemic capecitabine and oxaliplatin (XELOX) for gastric cancer with peritoneal metastases[J]. Ann Surg Oncol, 2023, 30(3): 1889-1890. |
| [13] | VERA G, CASTILLO M, CABEZOS P A, et al. Enteric neuropathy evoked by repeated cisplatin in the rat[J]. Neurogastroenterol Motil, 2011, 23(4): 370-378, e162-3. |
| [14] | WAFAI L, TAHER M, JOVANOVSKA V, et al. Effects of oxaliplatin on mouse myenteric neurons and colonic motility[J]. Front Neurosci, 2013, 7: 30. |
| [15] |
GORSCHLÜTER M, MEY U, STREHL J, et al. Invasive fungal infections in neutropenic enterocolitis: a systematic analysis of pathogens, incidence, treatment and mortality in adult patients[J]. BMC Infect Dis, 2006, 6: 35.
pmid: 16504141 |
| [16] |
MAROUN J A, ANTHONY L B, BLAIS N, et al. Prevention and management of chemotherapy-induced diarrhea in patients with colorectal cancer: a consensus statement by the Canadian Working Group on chemotherapy-induced diarrhea[J]. Curr Oncol, 2007, 14(1): 13-20.
doi: 10.3747/co.2007.96 pmid: 17576459 |
| [17] |
ARBUCKLE R B, HUBER S L, ZACKER C. The consequences of diarrhea occurring during chemotherapy for colorectal cancer: a retrospective study[J]. Oncologist, 2000, 5(3): 250-259.
pmid: 10884503 |
| [18] | ANDREYEV J, ROSS P, DONNELLAN C, et al. Guidance on the management of diarrhoea during cancer chemotherapy[J]. Lancet Oncol, 2014, 15(10): e447-60. |
| [19] | BOSSI P, ANTONUZZO A, CHERNY N I, et al. Diarrhoea in adult cancer patients: ESMO clinical practice guidelines[J]. Ann Oncol, 2018, 29(Suppl 4): iv126-iv142. |
| [20] | U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES. Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0[EB/OL]. [2024-10-15]. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf. |
| [21] |
BENSON A B 3rd, AJANI J A, CATALANO R B, et al. Recommended guidelines for the treatment of cancer treatment-induced diarrhea[J]. J Clin Oncol, 2004, 22(14): 2918-2926.
pmid: 15254061 |
| [22] |
HUSAIN A, APTAKER L, SPRIGGS D R, et al. Gastrointestinal toxicity and Clostridium difficile diarrhea in patients treated with paclitaxel-containing chemotherapy regimens[J]. Gynecol Oncol, 1998, 71(1): 104-107.
doi: 10.1006/gyno.1998.5158 pmid: 9784328 |
| [23] | SOOD N, CARBELL G, GREENWALD H S, et al. Is the medium still the message? Culture-independent diagnosis of gastrointestinal infections[J]. Dig Dis Sci, 2022, 67(1): 16-25. |
| [24] | National comprehensive cancer network. NCCN clinical practice guidelines in oncology (NCCN Guidelines). Hematopoietic Growth Factors, version 1.2025 [EB/OL]. [2024-10-15]. https://www.nccn.org/professionals/physician_gls/pdf/growthfactors.pdf. |
| [25] |
CARTONI C, DRAGONI F, MICOZZI A, et al. Neutropenic enterocolitis in patients with acute leukemia: prognostic significance of bowel wall thickening detected by ultrasonography[J]. J Clin Oncol, 2001, 19(3): 756-761.
pmid: 11157028 |
| [26] |
SCHNELL D, AZOULAY E, BENOIT D, et al. Management of neutropenic patients in the intensive care unit (NEWBORNS EXCLUDED) recommendations from an expert panel from the French Intensive Care Society (SRLF) with the French Group for Pediatric Intensive Care Emergencies (GFRUP), the French Society of Anesthesia and Intensive Care (SFAR), the French Society of Hematology (SFH), the French Society for Hospital Hygiene (SF2H), and the French Infectious Diseases Society (SPILF)[J]. Ann Intensive Care, 2016, 6(1): 90.
doi: 10.1186/s13613-016-0189-6 pmid: 27638133 |
| [27] | RUIZ-ARABI E, TORRE-CISNEROS J, AGUILERA V, et al. Management of cytomegalovirus in adult solid organ transplant patients: GESITRA-IC-SEIMC, CIBERINFEC, and SET recommendations update[J]. Transplant Rev (Orlando), 2024, 38(4): 100875. |
| [28] | MARASCO M, DELL’UNTO E, LAVIANO A, et al. Gastrointestinal side effects of somatostatin analogs in neuroendocrine tumors: a focused review[J]. J Gastroenterol Hepatol, 2024, 39(9): 1737-1744. |
| [29] |
KUNE G A, KUNE S, FIELD B, et al. The role of chronic constipation, diarrhea, and laxative use in the etiology of large-bowel cancer. data from the Melbourne colorectal cancer study[J]. Dis Colon Rectum, 1988, 31(7): 507-512.
pmid: 3391059 |
| [30] | WOOD L D, CANTO M I, JAFFEE E M, et al. Pancreatic cancer: pathogenesis, screening, diagnosis, and treatment[J]. Gastroenterology, 2022, 163(2): 386-402.e1. |
| [31] |
LI H, FU Z Y, ARSLAN M E, et al. Differential diagnosis and management of immune checkpoint inhibitor-induced colitis: a comprehensive review[J]. World J Exp Med, 2021, 11(6): 79-92.
doi: 10.5493/wjem.v11.i6.79 pmid: 36246150 |
| [32] | SUN K N, WANG X J, ZHANG H P, et al. Management and mechanisms of diarrhea induced by tyrosine kinase inhibitors in human epidermal growth factor receptor-2-positive breast cancer[J]. Cancer Control, 2024, 31: 10732748241278039. |
| [33] | LIU J N, YAN S, DU J T, et al. Mechanism and treatment of diarrhea associated with tyrosine kinase inhibitors[J]. Heliyon, 2024, 10(6): e27531. |
| [34] | DANIS R, MEGO M, ANTONOVA M, et al. Orally administered probiotics in the prevention of chemotherapy (± radiotherapy)-induced gastrointestinal toxicity: a systematic review with meta-analysis of randomized trials[J]. Integr Cancer Ther, 2022, 21: 15347354221144309. |
| [35] | EFREMOVA I, MASLENNIKOV R, POLUEKTOVA E, et al. Epidemiology of small intestinal bacterial overgrowth[J]. World J Gastroenterol, 2023, 29(22): 3400-3421. |
| [36] | GEE C, FLEURET C, WILSON A, et al. Bile acid malabsorption as a consequence of cancer treatment: prevalence and management in the national leading centre[J]. Cancers (Basel), 2021, 13(24): 6213. |
| [37] | XU Y S, XIONG L N, LI Y N, et al. Diagnostic methods and drug therapies in patients with ischemic colitis[J]. Int J Colorectal Dis, 2021, 36(1): 47-56. |
| [38] | SALVI F, PETRINO R, CONROY S P, et al. Constipation: a neglected condition in older emergency department patients[J]. Intern Emerg Med, 2024, 19(7): 1977-1986. |
| [39] |
HANEDA R, HIRAMATSU Y, KAWATA S, et al. Clinical impact of diarrhea during enteral feeding after esophagectomy[J]. Int J Clin Oncol, 2024, 29(1): 36-46.
doi: 10.1007/s10147-023-02428-5 pmid: 37994975 |
| [40] |
PETRELLI N, HERRERA L, RUSTUM Y, et al. A prospective randomized trial of 5-fluorouracil versus 5-fluorouracil and high-dose leucovorin versus 5-fluorouracil and methotrexate in previously untreated patients with advanced colorectal carcinoma[J]. J Clin Oncol, 1987, 5(10): 1559-1565.
doi: 10.1200/JCO.1987.5.10.1559 pmid: 2443619 |
| [41] | PETRELLI N, DOUGLASS H O Jr, HERRERA L, et al. The modulation of fluorouracil with leucovorin in metastatic colorectal carcinoma: a prospective randomized phase Ⅲ trial. Gastrointestinal tumor study group[J]. J Clin Oncol, 1989, 7(10): 1419-1426. |
| [42] | MEYERHARDT J A, MAYER R J. Systemic therapy for colorectal cancer[J]. N Engl J Med, 2005, 352(5): 476-487. |
| [43] | DEAN L, KANE M. Fluorouracil Therapy and DPYD Genotype[M]//PRATT V M, SCOTT S A, PIRMOHAMED M, et al. Medical Genetics Summaries. Bethesda (MD). 2012. |
| [44] | HAMAGUCHI T, SHIMADA Y, MIZUSAWA J, et al. Capecitabine versus S-1 as adjuvant chemotherapy for patients with stage Ⅲ colorectal cancer (JCOG0910): an open-label, non-inferiority, randomised, phase 3, multicentre trial[J]. Lancet Gastroenterol Hepatol, 2018, 3(1): 47-56. |
| [45] |
SLOAN J A, GOLDBERG R M, SARGENT D J, et al. Women experience greater toxicity with fluorouracil-based chemotherapy for colorectal cancer[J]. J Clin Oncol, 2002, 20(6): 1491-1498.
doi: 10.1200/JCO.2002.20.6.1491 pmid: 11896096 |
| [46] |
CASCINU S, BARNI S, LABIANCA R, et al. Evaluation of factors influencing 5-fluorouracil-induced diarrhea in colorectal cancer patients. An Italian Group for the study of digestive tract cancer (GISCAD) study[J]. Support Care Cancer, 1997, 5(4): 314-317.
pmid: 9257428 |
| [47] | INNOCENTI F. DPYD variants to predict 5-FU toxicity: the ultimate proof[J]. J Natl Cancer Inst, 2014, 106(12): dju351. |
| [48] |
MOREL A, BOISDRON-CELLE M, FEY L, et al. Clinical relevance of different dihydropyrimidine dehydrogenase gene single nucleotide polymorphisms on 5-fluorouracil tolerance[J]. Mol Cancer Ther, 2006, 5(11): 2895-2904.
doi: 10.1158/1535-7163.MCT-06-0327 pmid: 17121937 |
| [49] |
LEUNG H W C, CHAN A L F. Association and prediction of severe 5-fluorouracil toxicity with dihydropyrimidine dehydrogenase gene polymorphisms: a meta-analysis[J]. Biomed Rep, 2015, 3(6): 879-883.
pmid: 26623034 |
| [50] |
HE Y F, WEI W, ZHANG X, et al. Analysis of the DPYD gene implicated in 5-fluorouracil catabolism in Chinese cancer patients[J]. J Clin Pharm Ther, 2008, 33(3): 307-314.
doi: 10.1111/j.1365-2710.2008.00898.x pmid: 18452418 |
| [51] | 李婷婷, 谢颖, 何林海, 等. 基于5-氟尿嘧啶的直肠癌化疗疗效和不良反应的相关基因多态性研究进展[J]. 肿瘤研究与临床, 2019, 31(9): 644-648. |
| LI T T, XIE Y, HE L H, et al. Progress of related gene polymorphism of 5-fluorouracil-based chemotherapy efficacy and adverse reactions in colorectal cancer[J]. Cancer Res Clin, 2019, 31(9): 644-648. | |
| [52] | Clinical Pharmacogenetics Implementation Consortium. CPIC® Guideline for fluoropyrimidines and DPYD[EB/OL]. https://cpicpgx.org/guidelines/guideline-for-fluoropyrimidines-and-dpyd/. |
| [53] | KERR D J. Clinical efficacy of ‘Tomudex’ (raltitrexed) in advanced colorectal cancer[J]. Anticancer Drugs, 1997, 8(Suppl 2): S11-S15. |
| [54] |
DIASIO R B, BEAVERS T L, CARPENTER J T. Familial deficiency of dihydropyrimidine dehydrogenase. Biochemical basis for familial pyrimidinemia and severe 5-fluorouracil-induced toxicity[J]. J Clin Invest, 1988, 81(1): 47-51.
pmid: 3335642 |
| [55] | ABIGERGES D, CHABOT G G, ARMAND J P, et al. Phase Ⅰ and pharmacologic studies of the camptothecin analog irinotecan administered every 3 weeks in cancer patients[J]. J Clin Oncol, 1995, 13(1): 210-221. |
| [56] | XU S K, LAN H Y, HUANG C Y, et al. Mechanisms and emerging strategies for irinotecan-induced diarrhea[J]. Eur J Pharmacol, 2024, 974: 176614. |
| [57] |
SALIBA F, HAGIPANTELLI R, MISSET J L, et al. Pathophysiology and therapy of irinotecan-induced delayed-onset diarrhea in patients with advanced colorectal cancer: a prospective assessment[J]. J Clin Oncol, 1998, 16(8): 2745-2751.
pmid: 9704727 |
| [58] | FUCHS C S, MOORE M R, HARKER G, et al. Phase Ⅲ comparison of two irinotecan dosing regimens in second-line therapy of metastatic colorectal cancer[J]. J Clin Oncol, 2003, 21(5): 807-814. |
| [59] | HECHT J R. Gastrointestinal toxicity or irinotecan[J]. Oncology (Williston Park), 1998, 12(8Suppl 6): 72-78. |
| [60] | JIANG P C, WANG S W, LI C, et al. UGT1A1 genotype-guided irinotecan dosing during neoadjuvant chemoradiotherapy for locally advanced rectal cancer: a prospective analysis of SN-38 concentration[J]. Int J Cancer, 2024, 154(8): 1484-1491. |
| [61] | CHEN S J, HUA L, FENG C J, et al. Correlation between UGT1A1 gene polymorphism and irinotecan chemotherapy in metastatic colorectal cancer: a study from Guangxi Zhuang[J]. BMC Gastroenterol, 2020, 20(1): 96. |
| [62] | WANG Y, SHEN L, XU N, et al. UGT1A1 predicts outcome in colorectal cancer treated with irinotecan and fluorouracil[J]. World J Gastroenterol, 2012, 18(45): 6635-6644. |
| [63] | WASSERMAN E, MYARA A, LOKIEC F, et al. Severe CPT-11 toxicity in patients with Gilbert’s syndrome: two case reports[J]. Ann Oncol, 1997, 8(10): 1049-1051. |
| [64] | ZHANG M, WANG H W, HUANG Y C, et al. Compound heterozygous UGT1A1*28 and UGT1A1*6 or single homozygous UGT1A1*28 are major genotypes associated with Gilbert’s syndrome in Chinese Han people[J]. Gene, 2021, 781: 145526. |
| [65] |
TAKEDA Y, KOBAYASHI K, AKIYAMA Y, et al. Prevention of irinotecan (CPT-11)-induced diarrhea by oral alkalization combined with control of defecation in cancer patients[J]. Int J Cancer, 2001, 92(2): 269-275.
pmid: 11291056 |
| [66] | CHAMSEDDINE A N, DUCREUX M, ARMAND J P, et al. Intestinal bacterial β-glucuronidase as a possible predictive biomarker of irinotecan-induced diarrhea severity[J]. Pharmacol Ther, 2019, 199: 1-15. |
| [67] |
KWEEKEL D, GUCHELAAR H J, GELDERBLOM H. Clinical and pharmacogenetic factors associated with irinotecan toxicity[J]. Cancer Treat Rev, 2008, 34(7): 656-669.
doi: 10.1016/j.ctrv.2008.05.002 pmid: 18558463 |
| [68] | 中国临床肿瘤学会. 结直肠癌诊疗指南2024 [EB/OL]. (2024-08-13) [2024-10-15]. https://meeting.csco.org.cn/pdf/web/viewer.html?file=/Upload/Periodical/20240813105711.pdf. |
| Chinese Society of Clinical Oncology. Guidelines for the diagnosis and treatment of colorectal cancer 2024[EB/OL]. (2024-08-13) [2024-10-15]. https://meeting.csco.org.cn/pdf/web/viewer.html?file=/Upload/Periodical/20240813105711.pdf. | |
| [69] |
ROTHENBERG M L, MEROPOL N J, POPLIN E A, et al. Mortality associated with irinotecan plus bolus fluorouracil/leucovorin: summary findings of an independent panel[J]. J Clin Oncol, 2001, 19(18): 3801-3807.
doi: 10.1200/JCO.2001.19.18.3801 pmid: 11559717 |
| [70] |
FUCHS C S, MARSHALL J, BARRUECO J. Randomized, controlled trial of irinotecan plus infusional, bolus, or oral fluoropyrimidines in first-line treatment of metastatic colorectal cancer: updated results from the BICC-C study[J]. J Clin Oncol, 2008, 26(4): 689-690.
doi: 10.1200/JCO.2007.15.5390 pmid: 18235136 |
| [71] | COLUCCI G, GEBBIA V, PAOLETTI G, et al. Phase Ⅲ randomized trial of FOLFIRI versus FOLFOX4 in the treatment of advanced colorectal cancer: a multicenter study of the Gruppo Oncologico Dell’Italia Meridionale[J]. J Clin Oncol, 2005, 23(22): 4866-4875. |
| [72] | XU R H, MURO K, MORITA S, et al. Modified XELIRI (capecitabine plus irinotecan) versus FOLFIRI (leucovorin, fluorouracil, and irinotecan), both either with or without bevacizumab, as second-line therapy for metastatic colorectal cancer (AXEPT): a multicentre, open-label, randomised, non-inferiority, phase 3 trial[J]. Lancet Oncol, 2018, 19(5): 660-671. |
| [73] | FALCONE A, RICCI S, BRUNETTI I, et al. Phase Ⅲ trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as first-line treatment for metastatic colorectal cancer: the Gruppo Oncologico Nord Ovest[J]. J Clin Oncol, 2007, 25(13): 1670-1676. |
| [74] | 中国临床肿瘤学会. 胰腺癌诊疗指南2024 [EB/OL]. (2024-08-13) [2024-10-15] https://meeting.csco.org.cn/pdf/web/viewer.html?file=/Upload/Periodical/20240813112958.pdf. |
| Chinese Society of Clinical Oncology. Guidelines for the diagnosis and treatment of pancreatic cancer 2024[EB/OL].(2024-08-13) [2024-10-15] https://meeting.csco.org.cn/pdf/web/viewer.html?file=/Upload/Periodical/20240813112958.pdf. | |
| [75] | CHANG T C, SHIAH H S, YANG C H, et al. Phase Ⅰ study of nanoliposomal irinotecan (PEP02) in advanced solid tumor patients[J]. Cancer Chemother Pharmacol, 2015, 75(3): 579-586. |
| [76] |
ADIWIJAYA B S, KIM J, LANG I, et al. Population pharmacokinetics of liposomal irinotecan in patients with cancer[J]. Clin Pharmacol Ther, 2017, 102(6): 997-1005.
doi: 10.1002/cpt.720 pmid: 28445610 |
| [77] | WANG-GILLAM A, LI C P, BODOKY G, et al. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): a global, randomised, open-label, phase 3 trial[J]. Lancet, 2016, 387(10018): 545-557. |
| [78] | BANG Y J, LI C P, LEE K H, et al. Liposomal irinotecan in metastatic pancreatic adenocarcinoma in Asian patients: Subgroup analysis of the NAPOLI-1 study[J]. Cancer Sci, 2020, 111(2): 513-527. |
| [79] |
WAINBERG Z A, MELISI D, MACARULLA T, et al. NALIRIFOX versus nab-paclitaxel and gemcitabine in treatment-naive patients with metastatic pancreatic ductal adenocarcinoma (NAPOLI 3): a randomised, open-label, phase 3 trial[J]. Lancet, 2023, 402(10409): 1272-1281.
doi: 10.1016/S0140-6736(23)01366-1 pmid: 37708904 |
| [80] |
YOO C, KIM K P, JEONG J H, et al. Liposomal irinotecan plus fluorouracil and leucovorin versus fluorouracil and leucovorin for metastatic biliary tract cancer after progression on gemcitabine plus cisplatin (NIFTY): a multicentre, open-label, randomised, phase 2b study[J]. Lancet Oncol, 2021, 22(11): 1560-1572.
doi: 10.1016/S1470-2045(21)00486-1 pmid: 34656226 |
| [81] | BRENDEL K, BEKAII-SAAB T, BOLAND P M, et al. Population pharmacokinetics of liposomal irinotecan in patients with cancer and exposure-safety analyses in patients with metastatic pancreatic cancer[J]. CPT Pharmacometrics Syst Pharmacol, 2021, 10(12): 1550-1563. |
| [82] | ROY A C, PARK S R, CUNNINGHAM D, et al. A randomized phase Ⅱ study of PEP02 (MM-398), irinotecan or docetaxel as a second-line therapy in patients with locally advanced or metastatic gastric or gastro-oesophageal junction adenocarcinoma[J]. Ann Oncol, 2013, 24(6): 1567-1573. |
| [83] | CHIBAUDEL B, MAINDRAULT-GŒBEL F, BACHET J B, et al. PEPCOL: a GERCOR randomized phase Ⅱ study of nanoliposomal irinotecan PEP02 (MM-398) or irinotecan with leucovorin/5-fluorouracil as second-line therapy in metastatic colorectal cancer[J]. Cancer Med, 2016, 5(4): 676-683. |
| [84] | O’BRIEN B E, KAKLAMANI V G, BENSON A B 3rd. The assessment and management of cancer treatment-related diarrhea[J]. Clin Colorectal Cancer, 2005, 4(6): 375-381; discussion 382-383. |
| [85] | EADALA P, WAUD J P, MATTHEWS S B, et al. Quantifying the ‘hidden’ lactose in drugs used for the treatment of gastrointestinal conditions[J]. Aliment Pharmacol Ther, 2009, 29(6): 677-687. |
| [86] | Stanford Cancer Nutrition Services. Diarrhea nutrition tips[EB/OL]. (2024-08-13) [2024-10-15]. https://stanfordhealthcare.org/content/dam/SHC/programs-services/cancer-nutrition/docs/diarrhea-during-chemo-and-radiation-nutrition-facts.pdf. |
| [87] |
DUGGAN C, FONTAINE O, PIERCE N F, et al. Scientific rationale for a change in the composition of oral rehydration solution[J]. JAMA, 2004, 291(21): 2628-2631.
pmid: 15173155 |
| [88] |
RHODES A, EVANS L E, ALHAZZANI W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016[J]. Crit Care Med, 2017, 45(3): 486-552.
doi: 10.1097/CCM.0000000000002255 pmid: 28098591 |
| [89] |
ZAMPIERI F G, BAGSHAW S M, SEMLER M W. Fluid therapy for critically ill adults with sepsis: a review[J]. JAMA, 2023, 329(22): 1967-1980.
doi: 10.1001/jama.2023.7560 pmid: 37314271 |
| [90] |
MUSCARITOLI M, ARENDS J, BACHMANN P, et al. ESPEN practical guideline: clinical nutrition in cancer[J]. Clin Nutr, 2021, 40(5): 2898-2913.
doi: 10.1016/j.clnu.2021.02.005 pmid: 33946039 |
| [91] | OOMS L A, DEGRYSE A D, JANSSEN P A. Mechanisms of action of loperamide[J]. Scand J Gastroenterol Suppl, 1984, 96: 145-155. |
| [92] |
CASCINU S, BICHISAO E, AMADORI D, et al. High-dose loperamide in the treatment of 5-fluorouracil-induced diarrhea in colorectal cancer patients[J]. Support Care Cancer, 2000, 8(1): 65-67.
pmid: 10650901 |
| [93] |
EGGLESTON W, PALMER R, DUBÉ P A, et al. Loperamide toxicity: recommendations for patient monitoring and management[J]. Clin Toxicol (Phila), 2020, 58(5): 355-359.
doi: 10.1080/15563650.2019.1681443 pmid: 31684751 |
| [94] | LAMBERTS S W J, VAN DER LELY A J, DE HERDER W W, et al. Octreotide[J]. N Engl J Med, 1996, 334(4): 246-254. |
| [95] | DUENO M I, BAI J C, SANTANGELO W C, et al. Effect of somatostatin analog on water and electrolyte transport and transit time in human small bowel[J]. Dig Dis Sci, 1987, 32(10): 1092-1096. |
| [96] | MATON P N, O’DORISIO T M, HOWE B A, et al. Effect of a long-acting somatostatin analogue (SMS 201-995) in a patient with pancreatic cholera[J]. N Engl J Med, 1985, 312(1): 17-21. |
| [97] |
PETRELLI N J, RODRIGUEZ-BIGAS M, RUSTUM Y, et al. Bowel rest, intravenous hydration, and continuous high-dose infusion of octreotide acetate for the treatment of chemotherapy-induced diarrhea in patients with colorectal carcinoma[J]. Cancer, 1993, 72(5): 1543-1546.
pmid: 8348489 |
| [98] | WADLER S, HAYNES H, WIERNIK P H. Phase Ⅰ trial of the somatostatin analog octreotide acetate in the treatment of fluoropyrimidine-induced diarrhea[J]. J Clin Oncol, 1995, 13(1): 222-226. |
| [99] |
ZIDAN J, HAIM N, BENY A, et al. Octreotide in the treatment of severe chemotherapy-induced diarrhea[J]. Ann Oncol, 2001, 12(2): 227-229.
pmid: 11300329 |
| [100] |
BARBOUNIS V, KOUMAKIS G, VASSILOMANOLAKIS M, et al. Control of irinotecan-induced diarrhea by octreotide after loperamide failure[J]. Support Care Cancer, 2001, 9(4): 258-260.
pmid: 11430421 |
| [101] |
GOUMAS P, NAXAKIS S, CHRISTOPOULOU A, et al. Octreotide acetate in the treatment of fluorouracil-induced diarrhea[J]. Oncologist, 1998, 3(1): 50-53.
pmid: 10388084 |
| [102] |
CASCINU S, FEDELI A, FEDELI S L, et al. Octreotide versus loperamide in the treatment of fluorouracil-induced diarrhea: a randomized trial[J]. J Clin Oncol, 1993, 11(1): 148-151.
pmid: 8418225 |
| [103] | GEBBIA V, CARRECA I, TESTA A, et al. Subcutaneous octreotide versus oral loperamide in the treatment of diarrhea following chemotherapy[J]. Anticancer Drugs, 1993, 4(4): 443-445. |
| [104] |
CARDONA ZORRILLA A F, REVEIZ HERAULT L, CASASBUENAS A, et al. Systematic review of case reports concerning adults suffering from neutropenic enterocolitis[J]. Clin Transl Oncol, 2006, 8(1): 31-38.
doi: 10.1007/s12094-006-0092-y pmid: 16632437 |
| [105] | LIN X L, FANG Y, CHENG Y, et al. Chinese herbal medicine for irinotecan-induced diarrhea: a systematic review and meta-analysis[J]. Explore (NY), 2024, 20(2): 158-167. |
| [106] |
WU Y H, WANG D, YANG X Q, et al. Traditional Chinese medicine Gegen Qinlian decoction ameliorates irinotecan chemotherapy-induced gut toxicity in mice[J]. Biomed Pharmacother, 2019, 109: 2252-2261.
doi: S0753-3322(18)36907-5 pmid: 30551482 |
| [107] | 李玉凤, 张碧严, 赖芸, 等. 半夏泻心汤对氟尿嘧啶致腹泻小鼠模型肠道免疫功能的影响[J]. 中国实验方剂学杂志, 2014, 20(23): 180-184. |
| LI Y F, ZHANG B Y, LAI Y, et al. Effects of Banxia Xiexin Tang on intestinal immune function in fluorouracil-induced diarrhea mice[J]. Chin J Exp Tradit Med Formulae, 2014, 20(23): 180-184. | |
| [108] | TAKAHASHI T, NAGAI K, KOTAKE K. Efficacy of hangeshashinto in the prevention of chemotherapy-induced diarrhea: a systematic review and meta-analysis[J]. Cureus, 2023, 15(12): e50377. |
| [109] | ADAMS N, KUNENE V, MIKROPOULOS C. Management of systemic anti-cancer therapy induced diarrhoea in adult patients v2.3[EB/OL]. (2024-08-13) [2024-10-15]. https://www.england.nhs.uk/mids-east/wp-content/uploads/sites/7/2018/04/guidelines-for-management-of-diarrhoea-v2-3.pdf. |
| [110] | HOFF P M, SARAGIOTTO D F, BARRIOS C H, et al. Randomized phase Ⅲ trial exploring the use of long-acting release octreotide in the prevention of chemotherapy-induced diarrhea in patients with colorectal cancer: the LARCID trial[J]. J Clin Oncol, 2014, 32(10): 1006-1011. |
| [111] | VAN DEN HEUVEL B, PEETERS M, HENDLISZ A, et al. Long-acting octreotide as secondary prevention of chemotherapy-induced diarrhea: proof of concept[J]. Minerva Chir, 2016. |
| [112] |
ASTRUC B, MARBACH P, BOUTERFA H, et al. Long-acting octreotide and prolonged-release lanreotide formulations have different pharmacokinetic profiles[J]. J Clin Pharmacol, 2005, 45(7): 836-844.
doi: 10.1177/0091270005277936 pmid: 15951474 |
| [113] | OSTERLUND P, RUOTSALAINEN T, KORPELA R, et al. Lactobacillus supplementation for diarrhoea related to chemotherapy of colorectal cancer: a randomised study[J]. Br J Cancer, 2007, 97(8): 1028-1034. |
| [114] |
REDMAN M G, WARD E J, PHILLIPS R S. The efficacy and safety of probiotics in people with cancer: a systematic review[J]. Ann Oncol, 2014, 25(10): 1919-1929.
doi: S0923-7534(19)36598-6 pmid: 24618152 |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
沪ICP备12009617
Powered by Beijing Magtech Co. Ltd
