Welcome to China Oncology,
China Oncology ›› 2025, Vol. 35 ›› Issue (11): 1019-1031.doi: 10.19401/j.cnki.1007-3639.2025.11.004
• Article • Previous Articles Next Articles
ZHAO Jiaxuan1( ), WANG Yixuan1, TIAN Gaohui2, SHI Jiangzhou2, ZHANG Tongcun1,2(
), WANG Yixuan1, TIAN Gaohui2, SHI Jiangzhou2, ZHANG Tongcun1,2( )(
)( )
)
Received:2025-03-31
Revised:2025-08-21
Online:2025-11-30
Published:2025-12-12
Contact:
ZHANG Tongcun
E-mail:tony@tust.edu.cn
Supported by:Share article
CLC Number:
ZHAO Jiaxuan, WANG Yixuan, TIAN Gaohui, SHI Jiangzhou, ZHANG Tongcun. A study on optimization of the CAR-γδ T cell manufacturing process[J]. China Oncology, 2025, 35(11): 1019-1031.
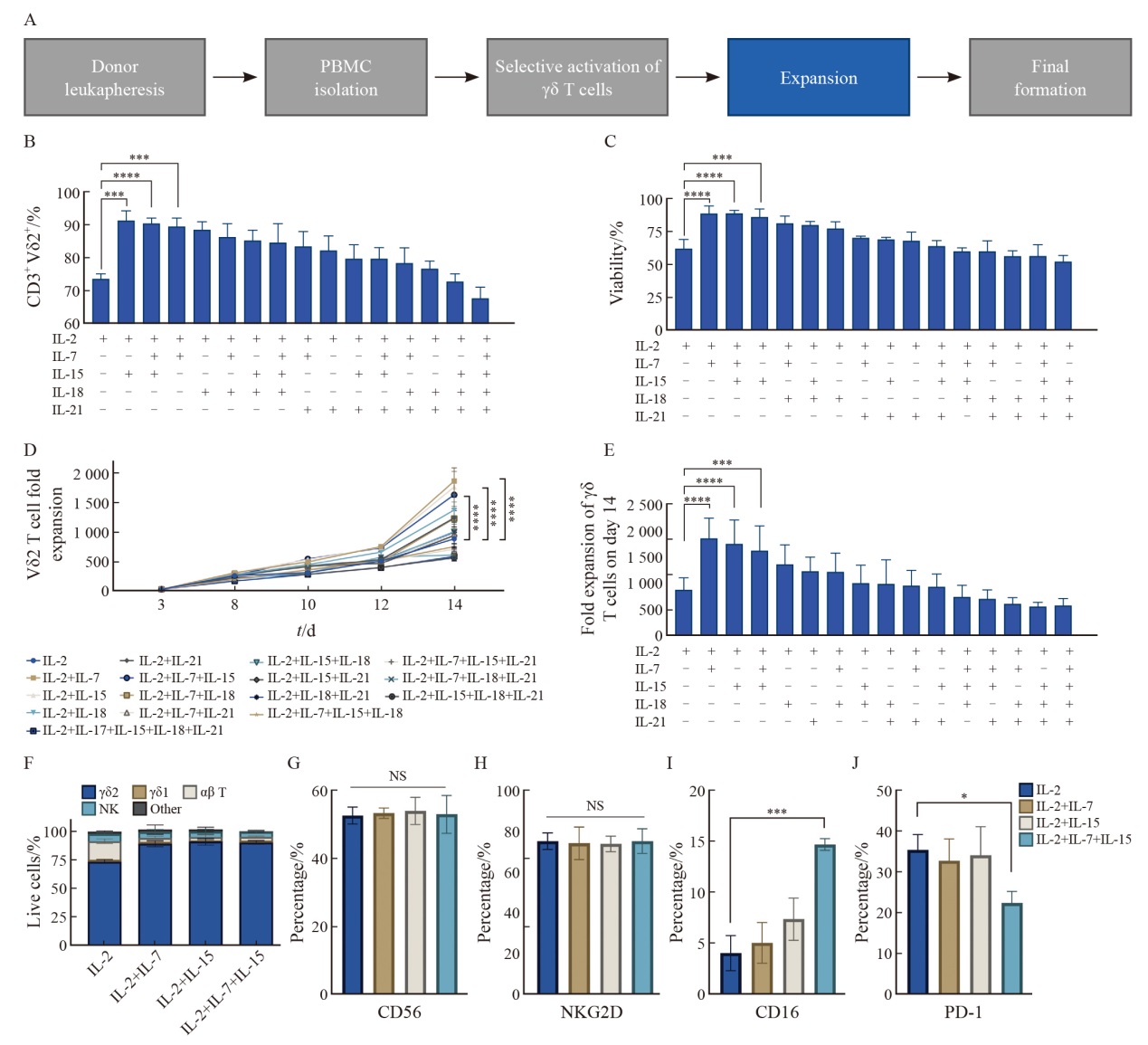
Fig. 1
The combination of IL-2, IL-7 and IL-15 enhances the expansion capacity and cytotoxic phenotype of γδ T cells while reducing exhaustion A: Flowchart of the γδ T cell production process. B: Purity of γδ T cells measured by flow cytometry on day 14 (n=3 donors). C: Summary of 7-AAD- live cells at day 14 (n=3 donors). D: Fold expansion of CD3+Vδ2+ T cells from day 0 to day 14 (n=3 donors). E: Fold expansion of CD3+Vδ2+ T cells on day 14 (n=3 donors). F: Summary of flow cytometry results comparing the expansion composition on day 14 after culture with IL-2+IL-7, IL-2+IL-15, IL-2+IL-7+IL-15, or IL-2 alone (n=3 donors). G-J: Expression levels of cytotoxicity markers CD56 (G), NKG2D (H), CD16 (I), and the exhaustion marker PD-1 (J) on γδ T cells (n=3 donors). Data are presented as $\bar{x} \pm s$. *: P<0.05; ***: P<0.001; ****: P<0.000 1. NS: Not significant. Statistical significance was determined by ANOVA."
Tab. 2
Summary of expansion data from cytokine combination screening"
| Group | Expansion fold | Cell purity | Cell viability |
|---|---|---|---|
| IL-2 | 876.50±238.35 | 73.67%±1.53% | 63.01%±7.05% |
| IL-2+IL-7 | 1 863.50±387.16 | 89.12%±3.61% | 89.33%±6.03% |
| IL-2+IL-15 | 1 763.50±452.33 | 91.33%±3.06% | 89.67%±6.11% |
| IL-2+IL-18 | 1 367.49±367.89 | 88.50%±2.50% | 77.67%±5.51% |
| IL-2+IL-21 | 1 233.50±269.59 | 82.33%±4.51% | 68.50%±6.56% |
| IL-2+IL-7+IL-15 | 1 627.50±472.15 | 90.69%±2.00% | 89.00%±3.61% |
| IL-2+IL-7+IL-18 | 1 221.50±359.75 | 86.33%±4.04% | 81.67%±5.86% |
| IL-2+IL-7+IL-21 | 955.50±292.85 | 83.50%±4.58% | 70.67%±1.15% |
| IL-2+IL-15+IL-18 | 1 000.50±359.16 | 85.33%±3.06% | 80.33%±3.06% |
| IL-2+IL-15+IL-21 | 932.50±254.44 | 79.67%±4.16% | 69.50%±1.50% |
| IL-2+IL-18+IL-21 | 988.50±462.76 | 76.67%±2.52% | 56.67%±3.51% |
| IL-2+IL-7+IL-15+IL-18 | 743.50±221.05 | 84.67%±5.86% | 60.33%±2.52% |
| IL-2+IL-7+IL-15+IL-21 | 700.50±177.34 | 79.67%±3.51% | 64.33%±4.04% |
| IL-2+IL-7+IL-18+IL-21 | 601.50±122.26 | 78.33%±4.73% | 60.33%±7.51% |
| IL-2+IL-15+IL-18+IL-21 | 554.50±83.35 | 71.33%±4.04% | 52.33%±4.51% |
| IL-2+IL-7+IL-15+IL-18+IL-21 | 578.50±132.25 | 67.50%±3.61% | 56.67%±8.33% |
Tab. 3
Summary of cell expansion data from IL-7 and IL-15 cytokine concentration optimization experiments"
| Group | Expansion fold | Cell purity | Cell viability |
|---|---|---|---|
| IL-2+IL-7LOW+IL-15LOW | 905.67±85.81 | 77.73%±1.35% | 75.90%±4.07% |
| IL-2+IL-7LOW+IL-15MEDIUM | 1 485.80±129.48 | 88.73%±3.62% | 89.10%±3.70% |
| IL-2+IL-7LOW+IL-15HIGH | 1 502.60±159.43 | 89.70%±1.90% | 81.97%±1.33% |
| IL-2+IL-7MEDIUM+IL-15LOW | 1 451.40±176.21 | 88.00%±1.76% | 90.47%±5.59% |
| IL-2+IL-7MEDIUM+IL-15MEDIUM | 1 465.53±123.03 | 88.77%±4.19% | 88.53%±2.80% |
| IL-2+IL-7MEDIUM+IL-15HIGH | 1 535.13±142.60 | 87.70%±2.69% | 81.27%±1.05% |
| IL-2+IL-7HIGH+IL-15LOW | 1 579.43±136.85 | 87.77%±3.32% | 87.47%±3.01% |
| IL-2+IL-7HIGH+IL-15MEDIUM | 1 454.23±184.48 | 86.97%±1.75% | 88.63%±5.74% |
| IL-2+IL-7HIGH+IL-15HIGH | 1 496.57±139.82 | 83.10%±2.39% | 80.30%±4.07% |
Tab. 4
Summary of cytotoxicity and exhaustion phenotype data from IL-7 and IL-15 cytokine concentration optimization experiments"
| Group | CD16+ | PD-1+ |
|---|---|---|
| IL-2+IL-7LOW+IL-15LOW | 6.57%±1.88% | 30.37%±7.72% |
| IL-2+IL-7LOW+IL-15MEDIUM | 12.63%±2.66% | 21.67%±5.37% |
| IL-2+IL-7LOW+IL-15HIGH | 15.53%±1.17% | 15.50%±4.04% |
| IL-2+IL-7MEDIUM+IL-15LOW | 7.35%±1.39% | 20.40%±7.14% |
| IL-2+IL-7MEDIUM+IL-15MEDIUM | 19.53%±4.52% | 15.27%±5.08% |
| IL-2+IL-7MEDIUM+IL-15HIGH | 19.77%±3.46% | 17.50%±3.85% |
| IL-2+IL-7HIGH+IL-15LOW | 10.69%±1.41% | 22.93%±5.93% |
| IL-2+IL-7HIGH+IL-15MEDIUM | 18.07%±1.36% | 17.17%±3.74% |
| IL-2+IL-7HIGH+IL-15HIGH | 16.93%±1.99% | 14.47%±3.59% |

Fig. 2
The concentration combination of IL-2+IL-7 (10 ng/mL)+IL-15 (10 ng/mL) significantly enhances the expansion capacity and cytotoxic phenotype of γδ T cells while reducing exhaustion A: Purity of γδ T cells measured by flow cytometry on day 14 (n=3 donors). B: Fold expansion of CD3+Vδ2+ T cells on day 14 (n=3 donors). C: Summary of 7-AAD- live cells at day 14 (n=3 donors). D, E: Representative flow cytometry plots of CD16+ and PD-1+ γδ T cells on day 14. F,G: Expression levels of cytotoxicity markers CD16+ and exhaustion marker PD-1+ γδ T cells on day 14. Data are presented as $\bar{x} \pm s$. *: P<0.05; **: P <0.01. NS: Not significant. Statistical significance was as determined by ANOVA (A,B) or Student’s t-test (C, F, G)."
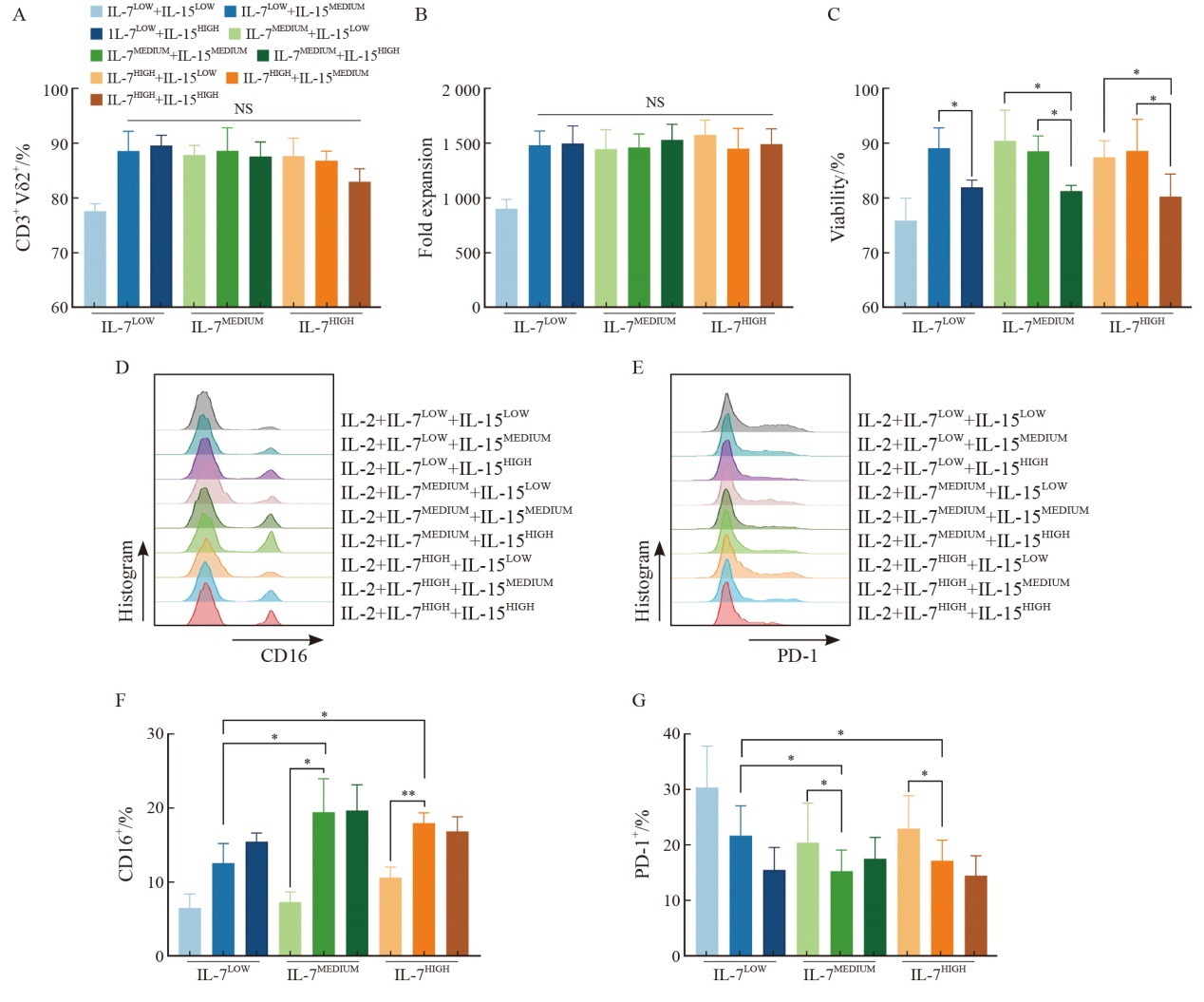
Fig. 3
γδ T cells activated for 96-120 h exhibit the optimal lentiviral transduction efficiency A: Flowchart of the γδ T cell production process. B: Schematic illustration of the EGFRvⅢ CAR construct. C: Representative flow cytometry plots of Strep Ⅱ-labeled CAR+ cells 6 d after transduction. D: Summary of transduction efficiency, CD25+CD69+ percentage, and CD3+Vδ2+ percentage of γδ T cells at different activation time points (n=3 donors). E: Correlation between transduction efficiency and CD25+CD69+ percentage. F: Correlation between transduction efficiency and CD3+Vδ2+ percentage in γδ T cells. Bars represent $\bar{x} \pm s$."
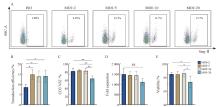
Fig. 4
CAR-γδ T cells transduced at MOI 5-10 exhibit optimal transduction efficiency and expansion capacity A: Representative flow cytometry plots showing Strep Ⅱ-labeled CAR+ 6 d after transduction. B: Quantification of transduction efficiency at varying MOIs (n=3 donors). C-E: Summary of purity, fold expansion and viability of EGFRvⅢ-γδ T cells on day 14. Data are presented as $\bar{x} \pm s$. *: P<0.05, **: P<0.01. NS: Not significant."
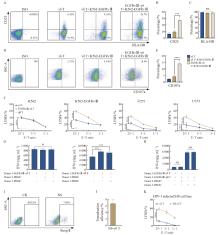
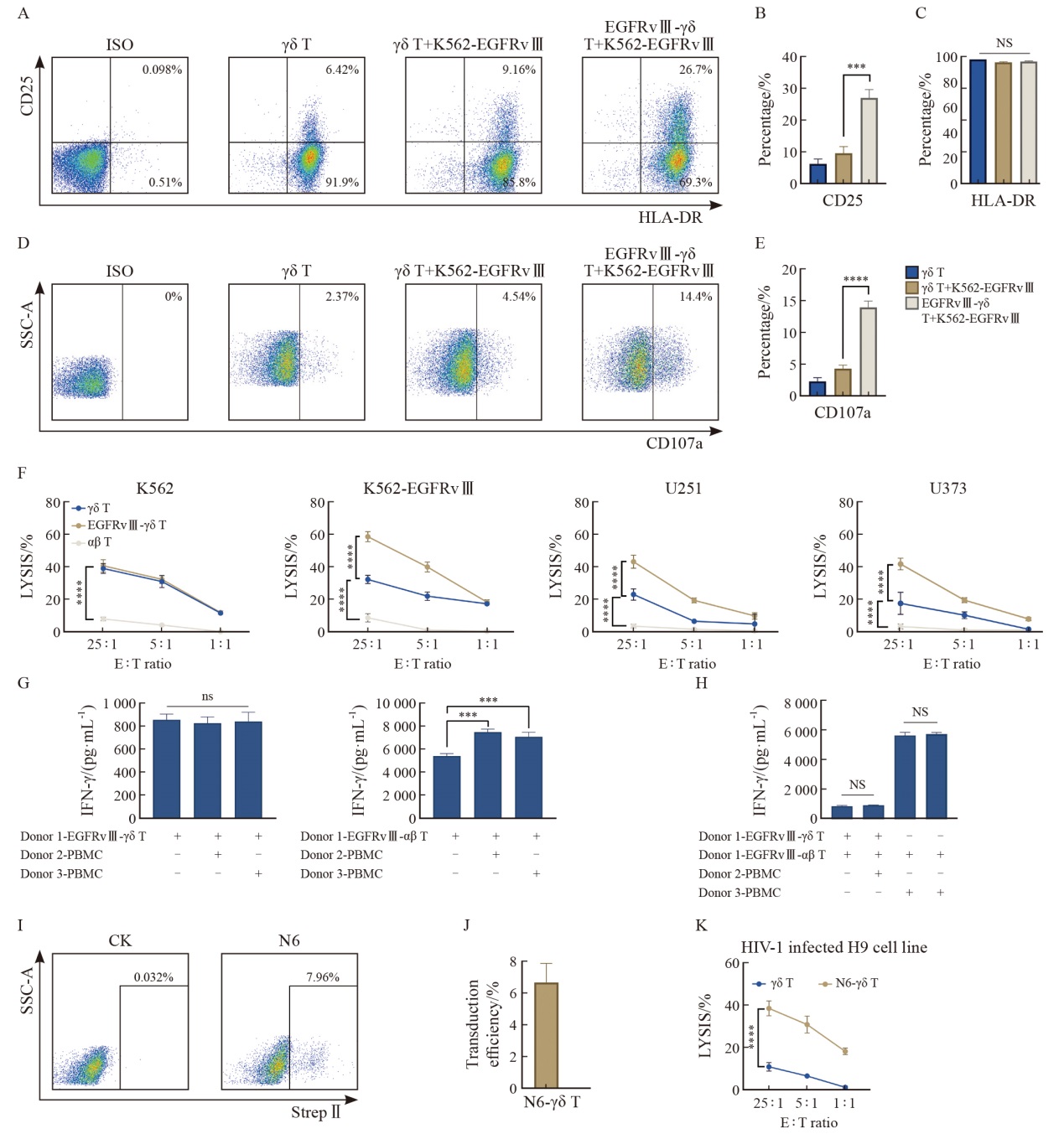
Fig. 5
CAR-γδ T cells cultured by optimal conditions demonstrate enhanced cytotoxicity with preliminarily no evidence of GVHD A: representative flow cytometry plots of CD25 and HLA-DR. B, C: Summarized results (n=3 donors) of CD25 and HLA-DR expression on γδ T or CAR-γδ T cells. D: Representative flow cytometry plots of CD107a. E: Summarized results (n=3 donors) of CD107a expression on γδ T or CAR-γδ T cells. F: Cytotoxic activity of CAR-γδ T cells against four cell lines, as determined by calcein release assay at different E∶T ratios (1∶1, 5∶1, 25∶1) after 3.5 h of co-culture (one representative donor from two is shown; n=4 independent wells). G, H: Detection of IFN-γ in the supernatants from EGFRvⅢ-γδ T and EGFRvⅢ-αβ T cells co-cultured with other donors’ PBMC and own PBMC (n=3 independent wells). I: Representative flow cytometry plots of Strep Ⅱ-labeled CAR+ 6 days after transduction. J: Summarized results (n=3 donors) of Transduction efficiency of N6-γδ T cells. K: The H9 cell line was infected with HIV-1 (NL4-3) and mixed with N6 CAR-γδ T cells at different ratios (1∶1, 5∶1, 25∶1). Direct cytotoxicity effects were detected (one representative donor from two is shown; n=4 independent wells). Data are presented as $\bar{x} \pm s$. ***: P<0.001; ****: P <0.000 1. NS: Not significant."
| [1] |
ISMAIL F S, GALLUS M, MEUTH S G, et al. Current and future roles of chimeric antigen receptor T-cell therapy in neurology: a review[J]. JAMA Neurol, 2025, 82(1): 93-103.
doi: 10.1001/jamaneurol.2024.3818 pmid: 39585688 |
| [2] |
LV J Z, LIU Z, REN X T, et al. γδ T cells, a key subset of T cell for cancer immunotherapy[J]. Front Immunol, 2025, 16: 1562188.
doi: 10.3389/fimmu.2025.1562188 |
| [3] | HU Y, HU Q L, LI Y S, et al. γδ T cells: origin and fate, subsets, diseases and immunotherapy[J]. Signal Transduct Target Ther, 2023, 8(1): 434. |
| [4] |
LU Y W, XIANG Z Q, WANG W J, et al. Establishment and validation of a tumor-infiltrating γδ T cell related prognostic gene signature in head and neck squamous cell carcinoma[J]. Int Immunopharmacol, 2024, 132: 112054.
doi: 10.1016/j.intimp.2024.112054 |
| [5] |
LI W J, ZHAO X, REN C X, et al. The therapeutic role of γδ T cells in TNBC[J]. Front Immunol, 2024, 15: 1420107.
doi: 10.3389/fimmu.2024.1420107 |
| [6] |
ROZENBAUM M, MEIR A, AHARONY Y, et al. Gamma-delta CAR-T cells show CAR-directed and independent activity against leukemia[J]. Front Immunol, 2020, 11: 1347.
doi: 10.3389/fimmu.2020.01347 pmid: 32714329 |
| [7] |
SUMARIA N, ROEDIGER B, NG L G, et al. Cutaneous immunosurveillance by self-renewing dermal gammadelta T cells[J]. J Exp Med, 2011, 208(3): 505-518.
doi: 10.1084/jem.20101824 |
| [8] | LANDIN A M, COX C, YU B, et al. Expansion and enrichment of gamma-delta (γδ) T cells from apheresed human product[J]. J Vis Exp, 2021, (175): e62622. |
| [9] |
WANG H, WANG X L, WANG W, et al. Interleukin-15 enhanced the survival of human γδ T cells by regulating the expression of Mcl-1 in neuroblastoma[J]. Cell Death Discov, 2022, 8: 139.
doi: 10.1038/s41420-022-00942-5 |
| [10] |
KHAN M W A, OTAIBI A A, SHERWANI S, et al. Optimization of methods for peripheral blood mononuclear cells isolation and expansion of human gamma delta T cells[J]. Bioinformation, 2021, 17(3): 460-469.
doi: 10.6026/97320630017460 pmid: 34092966 |
| [11] |
TEO H Y, SONG Y, YONG K S M, et al. IL12/18/21 preactivation enhances the antitumor efficacy of expanded γδ T cells and overcomes resistance to anti-PD-L1 treatment[J]. Cancer Immunol Res, 2023, 11(7): 978-999.
doi: 10.1158/2326-6066.CIR-21-0952 |
| [12] |
SONG Y, TEO H Y, LIU Y H, et al. Reviving human γδ T cells from apoptosis induced by IL-12/18 via p-JNK inhibition[J]. J Leukoc Biol, 2022, 112(6): 1701-1716.
doi: 10.1002/JLB.5MA0622-741R |
| [13] | TYLER C J, HOTI I, GRIFFITHS D D, et al. IL-21 conditions antigen-presenting human γδ T-cells to promote IL-10 expression in naïve and memory CD4+ T-cells[J]. Discov Immunol, 2024, 3(1): kyae008. |
| [14] |
ISHIKAWA J, SUTO A, ABE K, et al. IL-21 is required for the maintenance and pathogenesis of murine Vγ4+ IL-17-producing γδ T cells[J]. Front Immunol, 2023, 14: 1211620.
doi: 10.3389/fimmu.2023.1211620 |
| [15] |
STITZ J. Development of HIV-1 vectors pseudotyped with envelope proteins of other retroviruses[J]. Virology, 2025, 602: 110300.
doi: 10.1016/j.virol.2024.110300 |
| [16] | WANG R N, WEN Q, HE W T, et al. Optimized protocols for γδ T cell expansion and lentiviral transduction[J]. Mol Med Rep, 2019, 19(3): 1471-1480. |
| [17] | KONDO M, IZUMI T, FUJIEDA N, et al. Expansion of human peripheral blood γδ T cells using zoledronate[J]. J Vis Exp, 2011(55): 3182. |
| [18] |
SATO K, KONDO M, SAKUTA K, et al. Impact of culture medium on the expansion of T cells for immunotherapy[J]. Cytotherapy, 2009, 11(7): 936-946.
doi: 10.3109/14653240903219114 pmid: 19903105 |
| [19] |
AEHNLICH P, CARNAZ SIMÕES A M, SKADBORG S K, et al. Expansion with IL-15 increases cytotoxicity of Vγ9Vδ2 T cells and is associated with higher levels of cytotoxic molecules and T-bet[J]. Front Immunol, 2020, 11: 1868.
doi: 10.3389/fimmu.2020.01868 pmid: 32983105 |
| [20] |
YOUNAS M, HUE S, LACABARATZ C, et al. IL-7 modulates in vitro and in vivo human memory T regulatory cell functions through the CD39/ATP axis[J]. J Immunol, 2013, 191(6): 3161-3168.
doi: 10.4049/jimmunol.1203547 |
| [21] |
RATHMELL J C, FARKASH E A, GAO W, et al. IL-7 enhances the survival and maintains the size of naive T cells[J]. J Immunol, 2001, 167(12): 6869-6876.
doi: 10.4049/jimmunol.167.12.6869 pmid: 11739504 |
| [22] |
KING L A, JONG M D, VETH M, et al. Vδ2 T-cell engagers bivalent for Vδ2-TCR binding provide anti-tumor immunity and support robust Vγ9Vδ2 T-cell expansion[J]. Front Oncol, 2024, 14: 1474007.
doi: 10.3389/fonc.2024.1474007 |
| [23] |
PETERS C, HÄSLER R, WESCH D, et al. Human Vδ2 T cells are a major source of interleukin-9[J]. Proc Natl Acad Sci USA, 2016, 113(44): 12520-12525.
pmid: 27791087 |
| [24] |
VAN ACKER H H, ANGUILLE S, WILLEMEN Y, et al. Interleukin-15 enhances the proliferation, stimulatory phenotype, and antitumor effector functions of human gamma delta T cells[J]. J Hematol Oncol, 2016, 9(1): 101.
doi: 10.1186/s13045-016-0329-3 |
| [25] |
SCHILBACH K, WELKER C, KRICKEBERG N, et al. In the absence of a TCR signal IL-2/IL-12/18-stimulated γδ T cells demonstrate potent anti-tumoral function through direct killing and senescence induction in cancer cells[J]. Cancers (Basel), 2020, 12(1): 130.
doi: 10.3390/cancers12010130 |
| [26] |
THEDREZ A, HARLY C, MORICE A, et al. IL-21-mediated potentiation of antitumor cytolytic and proinflammatory responses of human V gamma 9V delta 2 T cells for adoptive immunotherapy[J]. J Immunol, 2009, 182(6): 3423-3431.
doi: 10.4049/jimmunol.0803068 pmid: 19265120 |
| [27] |
NOVY P, HUANG X P, LEONARD W J, et al. Intrinsic IL-21 signaling is critical for CD8 T cell survival and memory formation in response to vaccinia viral infection[J]. J Immunol, 2011, 186(5): 2729-2738.
doi: 10.4049/jimmunol.1003009 pmid: 21257966 |
| [28] |
BESSER M J, SCHALLMACH E, OVED K, et al. Modifying interleukin-2 concentrations during culture improves function of T cells for adoptive immunotherapy[J]. Cytotherapy, 2009, 11(2): 206-217.
doi: 10.1080/14653240802590391 pmid: 19148842 |
| [29] |
GHASSEMI S, DURGIN J S, NUNEZ-CRUZ S, et al. Rapid manufacturing of non-activated potent CAR T cells[J]. Nat Biomed Eng, 2022, 6(2): 118-128.
doi: 10.1038/s41551-021-00842-6 pmid: 35190680 |
| [30] |
NOAKS E, PETICONE C, KOTSOPOULOU E, et al. Enriching leukapheresis improves T cell activation and transduction efficiency during CAR T processing[J]. Mol Ther Methods Clin Dev, 2021, 20: 675-687.
doi: 10.1016/j.omtm.2021.02.002 |
| [31] |
李帆, 张琴星, 童祥文, 等. 不同信号肽对嵌合抗原受体T细胞杀伤作用的影响研究[J]. 中国癌症杂志, 2022, 32(2): 142-151.
doi: 10.19401/j.cnki.1007-3639.2022.02.006 |
|
LI F, ZHANG Q X, TONG X W, et al. A study on influence of different signal peptides on anti-tumor effect of chimeric antigen receptor (CAR) T cells[J]. Chin Oncol, 2022, 32(2): 142-151.
doi: 10.19401/j.cnki.1007-3639.2022.02.006 |
|
| [32] |
ZHANG S K, GU C J, HUANG L F, et al. The third-generation anti-CD30 CAR T-cells specifically homing to the tumor and mediating powerful antitumor activity[J]. Sci Rep, 2022, 12(1): 10488.
doi: 10.1038/s41598-022-14523-0 pmid: 35729339 |
| [33] |
SHITARA S, HARA T, LIANG B F, et al. IL-7 produced by thymic epithelial cells plays a major role in the development of thymocytes and TCRγδ+ intraepithelial lymphocytes[J]. J Immunol, 2013, 190(12): 6173-6179.
doi: 10.4049/jimmunol.1202573 |
| [34] |
BACCALA R, WITHERDEN D, GONZALEZ-QUINTIAL R, et al. Gamma delta T cell homeostasis is controlled by IL-7 and IL-15 together with subset-specific factors[J]. J Immunol, 2005, 174(8): 4606-4612.
pmid: 15814683 |
| [35] |
田高辉, 张琴星, 史江舟, 等. 靶向CD30的CAR-T细胞慢病毒转导条件优化研究[J]. 中国癌症杂志, 2023, 33(7): 646-654.
doi: 10.19401/j.cnki.1007-3639.2023.07.002 |
|
TIAN G H, ZHANG Q X, SHI J Z, et al. A study on optimized lentiviral transduction conditions in CAR-T cells targeting CD30[J]. Chin Oncol, 2023, 33(7): 646-654.
doi: 10.19401/j.cnki.1007-3639.2023.07.002 |
|
| [36] |
CAVALIERI S, CAZZANIGA S, GEUNA M, et al. Human T lymphocytes transduced by lentiviral vectors in the absence of TCR activation maintain an intact immune competence[J]. Blood, 2003, 102(2): 497-505.
doi: 10.1182/blood-2003-01-0297 pmid: 12649146 |
| [37] | LANITIS E, ROTA G, KOSTI P, et al. Optimized gene engineering of murine CAR-T cells reveals the beneficial effects of IL-15 coexpression[J]. J Exp Med, 2021, 218(2): e20192203. |
| [38] |
DANOVI S. Identifying regulators of γδ T cell activity[J]. Nat Genet, 2023, 55(11): 1781.
doi: 10.1038/s41588-023-01579-5 pmid: 37938721 |
| [39] |
LO PRESTI V, CORNEL A M, PLANTINGA M, et al. Efficient lentiviral transduction method to gene modify cord blood CD8+ T cells for cancer therapy applications[J]. Mol Ther Methods Clin Dev, 2021, 21: 357-368.
doi: 10.1016/j.omtm.2021.03.015 |
| [1] | LI Fan, ZHANG Qinxing, TONG Xiangwen, TIAN Gaohui, GU Lixing, XU Yao. A study on influence of different signal peptides on anti-tumor effect of chimeric antigen receptor (CAR) T cells [J]. China Oncology, 2022, 32(2): 142-151. |
| [2] | LIU Xiao-jun,WANG Na,YAO Xu-dong,CAO Da-long. Construction of eukaryotic expression vector and package of lentivirus vector encoding prostate cancer antigen 3 [J]. China Oncology, 2013, 23(11): 857-862. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
沪ICP备12009617
Powered by Beijing Magtech Co. Ltd
