Welcome to China Oncology,
China Oncology ›› 2025, Vol. 35 ›› Issue (10): 906-919.doi: 10.19401/j.cnki.1007-3639.2025.10.002
• Specialist's Commentary • Previous Articles Next Articles
LAN Xinyue( ), ZHOU Yicheng, CHEN Dongqin(
), ZHOU Yicheng, CHEN Dongqin( )
)
Received:2025-06-24
Revised:2025-09-16
Online:2025-10-30
Published:2025-11-19
Contact:
CHEN Dongqin
Share article
CLC Number:
LAN Xinyue, ZHOU Yicheng, CHEN Dongqin. Survival impact of corticosteroid and immunosuppressant management strategies for immune-related adverse events in immune checkpoint inhibitor-treated patients: a systematic review and meta-analysis[J]. China Oncology, 2025, 35(10): 906-919.
Tab. 1
Characteristics of included studies"
| Study | Country | Study design | Sample size | Cancer | Immunotherapy | Outcome | NOS |
|---|---|---|---|---|---|---|---|
| Bai, et al [ | America, Australia | Multi-center retrospective cohort study | 947 | Melanoma | Nivolumab, pembrolizumab | PFS, post-irAE OS, post-irAE PFS | 8 |
| Bruyère, et al [ | France | Single-center retrospective cohort study | 828 | Various | Anti-PD-(L)1 (90%) Anti-CTLA-4 (10%) | OS, PFS | 7 |
| Shimomura, et al [ | Japan | Single-center retrospective cohort study | 172 | NSCLC | nivolumab pembrolizumab | OS, TTF | 7 |
| Matsukane, et al [ | Japan | Single-center retrospective cohort study | 935 | Various | Nivolumab, pembrolizumab, durvalumab, atezolizumab, avelumab | OS | 6 |
| Cariou, et al [ | France | Single-center prospective cohort study | 184 | Various | Nivolumab, pembrolizumab, nivolumab + ipilimumab | OS, PFS | 8 |
| Robesti, et al [ | Multiple countries | Post hoc analysis of RCT | 896 | UC | Atezolizumab | OS, PFS, CSS | 9 |
| Syed, et al [ | America | Single-center retrospective case-control study | 167 | NSCLC Melanoma | Anti-PD-(L)1, Anti-CTLA-4 | OS, PFS | 8 |
| Verheijden, et al [ | Multiple countries | Multi-center retrospective cohort study | 606 | Melanoma | Anti-PD-1 (33%), Anti-PD-1+ anti-CTLA-4 (67%) | Post-irAE OS, post-irAE PFS | 9 |
| Curkovic, et al [ | America | Multi-center retrospective cohort study | 226 | Melanoma | Nivolumab + ipilimumab | OS, PFS | 9 |
| Pichler, et al [ | Multiple countries | Multi-center retrospective cohort study | 335 | UC | Pembrolizumab, nivolumab, atezolizumab, avelumab | OS, PFS | 8 |
| Verheijden, et al [ | Multiple countries | Post hoc analysis of RCT | 1 959 | Various | Nivolumab+ipilimumab | OS, PFS, post-irAE OS, post-irAE PFS | 9 |
Tab. 2
Corticosteroids and immunosuppressants use characteristics of included studies"
| Study | Sample details | CS type | Timing of initiation | Peak dose | Cumulative dose | Treatment duration | IM co-administration |
|---|---|---|---|---|---|---|---|
| Bai, et al[ | irAE n=509 CS group (peak dose >30 mg prednisone equivalent per day): n=164 | Prednisone-equivalent only | Stratified: ≤8 weeks vs >8 weeks; ≤26 weeks vs >26 weeks | Stratified: High dose (≥60 mg/d) vs low dose (<60 mg/d) | Not reported | Median tapering duration: 6 days | Not reported |
| Bruyère, et al[ | irAE n=78 (grade ≥3) CS group: n=45; CS+IM group: n=8 | Prednisone-equivalent only | Not reported | High-dose corticosteroids ≥1 mg/kg | Not reported | Median CTLA-4: 42 d; PD-1: 47 d | Incomplete reporting |
| Shimomura, et al[ | irAE n=144; CS group: n=66 | Prednisone-equivalent only | Time from ICI initiation to corticosteroid use. Median: High dose: 84 d; Low dose: 91 d; Stratified: ≤60 d vs >60 d | Stratified: High dose (≥0.5 mg/kg) vs no corticosteroids Low dose (<0.5 mg/kg) vs no corticosteroids | Not reported | Median: High dose: 14 d; Low dose: 90 d | Not reported |
| Matsukane, et al[ | irAE n=400; CS group: n=170 | Prednisone-equivalent only | Not reported | No-corticosteroid administration: Low dose (< 0.5 mg/ kg), moderate to high dose (0.5-2.0 mg/ kg), intravenous methylprednisolone pulse therapy (500-1 000 mg, 3 days). Stratified: Intravenous methylprednisolone pulse therapy vs no pulse therapy | Not reported | Not reported | Not reported |
| Cariou, et al[ | irAE n=184; CS group: 107; CS+IM group: n=20 | Prednisone-equivalent only | Time from ICI initiation to corticosteroid use. Median (95% CI): CS group: 4.5 (1.6-9.1) months CS+IM group: 3.4 (2.0-9.3) months | Incomplete reporting | Not reported | Median (95% CI): CS group: 3.1 (2.7-4.4) months; CS+IM group: 7.1 (4.3-29.0) months | 20 patients received anti-TNF alpha agents, cyclophosphamide, methotrexate, azathioprine |
| Robesti, et al[ | irAE n=195 CS group: n=99 | Prednisone-equivalent only | Not reported | Incomplete reporting Stratified: CS alone use vs no use | Not reported | Not reported | Not reported |
| Syed, et al[ | irAE n=167 CS group: n=132 CS+IM group: n=35 | Prednisone-equivalent only | Time from ICI initiation to corticosteroid use. Median (IQR): CS group: 2.99 (1.56, 7.84) months; CS+IM group: 2.77 (1.23, 8.13) months | Not reported | Not reported | Median (IQR): CS group: 2.17 (1.50, 4.00) months; CS+IM group: 3.00 (2.00, 5.08) months | Mycophenolate mofetil 15 (42.9%) patients, infliximab 12 (34.3%) patients, rituximab 3 (8.6%) patients, tocilizumab 2 (5.7%) patients, methotrexate 2 patients (5.7%), tacrolimus 2 (5.7%) patients; Stratified: CS+IM use vs CS alone use |
| Verheijden, et al[ | irAE n=606 CS group: 425 CS+IM group: 181 | Prednisone-equivalent only | Time time from irAE onset to immunosuppression use. Median: 2 days | Median (IQR): CS group: 80 (60-107) mg; CS+IM group: 110 (80-160) mg; Stratified: 160 mg vs 40 mg | Median (IQR): 1 503-3 743 mg; CS+IM group: 3 900 (2 483-5 684) mg | Not reported | TNF inhibition 102 (56%) patients, mycophenolate mofetyl 59 (33%) patients, tacrolimus 22 (12%) patients, intravenous immunoglobulins (IVIg) 20 (11%) patients; vedolizumab 9 (5%) patients, methotrexate 5 (3%) patients, others 11 (6%) patients; Stratified: CS+IM use vs CS alone use |
| Curkovic, et al[ | irAE n=183 CS group: n=136 CS+IM group: NS | Prednisone-equivalent only | Time from ICI initiation to corticosteroid. Median (IQR): 49 (28-70.2) days Stratified: Per-day delay in CS initiation | Median (IQR): 80 (60-150) days; Stratified: Per 1 mg increase in peak dose | Incomplete reporting Stratified: Per 1 mg increase in cumulative dose | Not reported | Incomplete reporting; Stratified: CS+IM use vs CS alone use |
| Pichler, et al[ | irAE n=122 CS group: n=62 | Prednisone-equivalent only | Not reported | Incomplete reporting; Stratified: CS alone use vs no use | Not reported | Not reported | Not reported |
| Verheijden, et al[ | irAE n=834 CS group: n=753 CS+IM group: n=79 | Prednisone-equivalent only | 50% within 1 day of irAE onset and 82% started within 1 week | Median (IQR): 75 (40-125) mg, which corresponded to 1.0 mg/kg (IQR, 0.54-1.51 mg/kg); Stratified: 0.5 vs 1.0 vs 2.0 mg/kg | Median (IQR): 1 850 (680-3 712) mg Stratified: Per 1 000 mg increase in cumulative dose | Median (IQR): 54 (20-102) days | Tumor necrosis factor (TNF) inhibition 44 (5%) patients; mycophenolate mofetil 22 (3%) patients; intravenous immunoglobulins (IVIg) 14 (2%) patients; others 9 (1%) patients; Stratified: CS+IM use vs CS alone use |

Fig. 3
Forest plots evaluating the effect of cumulative corticosteroid dose on post-irAE OS and PFS of the patients MSI-H/dMMR CRC: Microsatellite instability-high/mismatch repair-deficient colorectal cancer; NSCLC: Non-small cell lung cancer. A: Effect of cumulative corticosteroid dose on post-irAE OS; B: Effect of cumulative corticosteroid dose on post-irAE PFS"
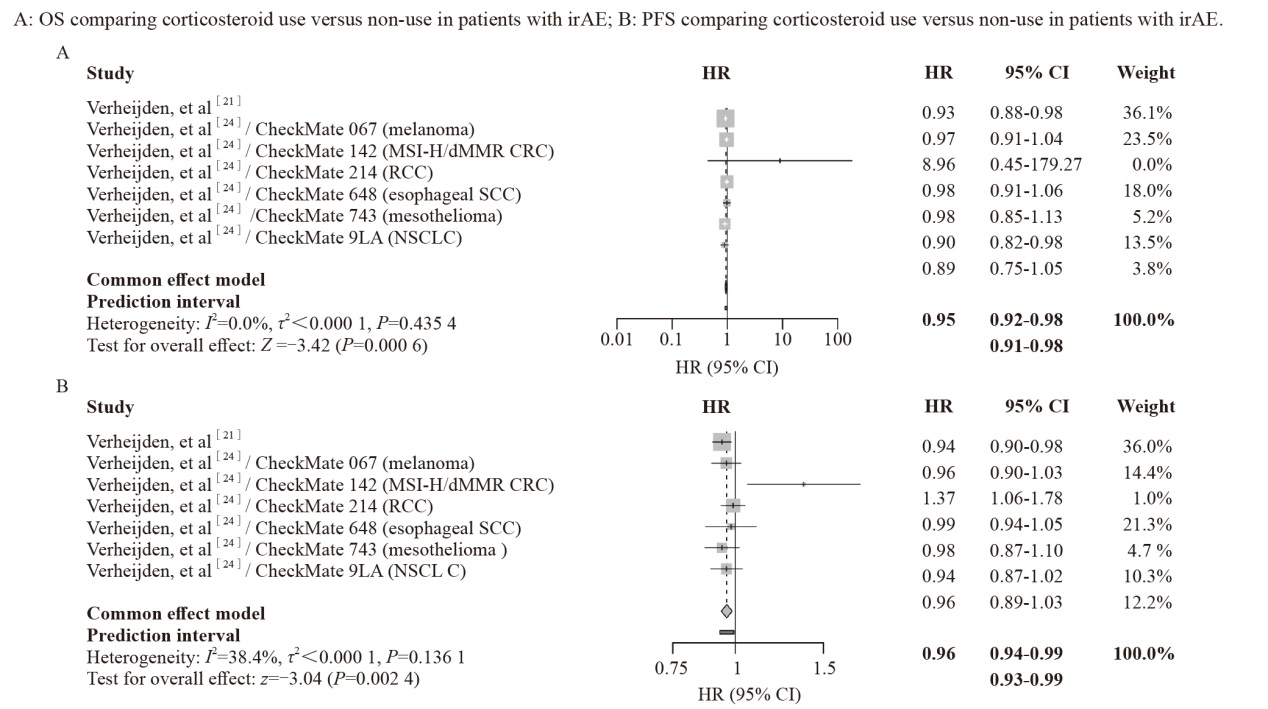
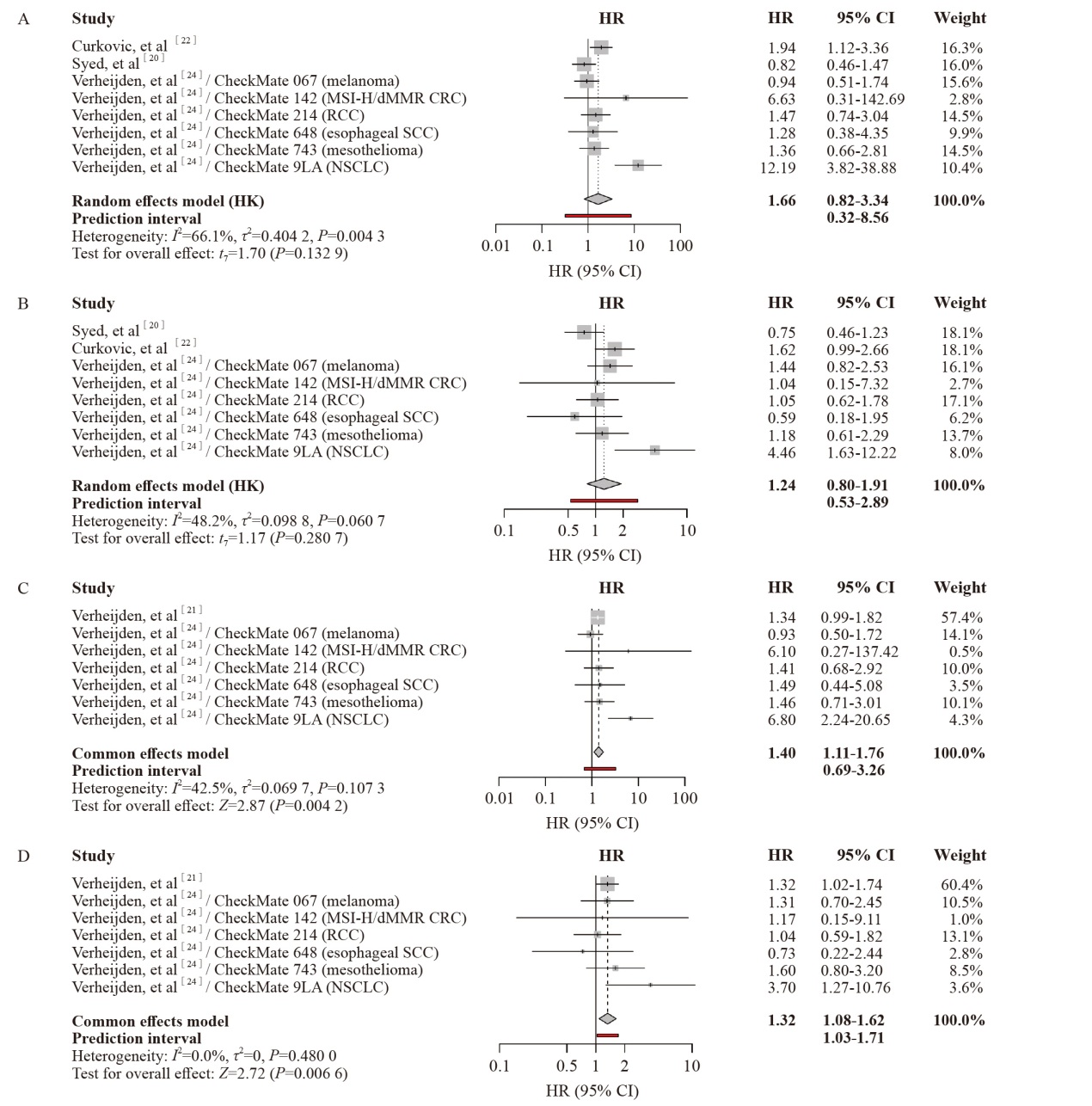
Fig. 4
Forest plots comparing second-line immunosuppressant (IM) use versus corticosteroid alone on OS and PFS of the patients MSI-H/dMMR CRC: Microsatellite instability-high/mismatch repair-deficient colorectal cancer; NSCLC: Non-small cell lung cancer. A: Effect of second-line immunosuppressant use on OS; B: Effect of second-line immunosuppressant use on PFS; C: Effect of second-line immunosuppressant use on post-irAE OS; D: Effect of second-line immunosuppressant use on post-irAE PFS."
Tab. 3
Results of publication bias analysis"
| Risk factor | n | Egger's regression P value | Begg's rank correlation P value |
|---|---|---|---|
| Corticosteroid use vs non-use (OS) | 2 | - | - |
| Corticosteroid use vs non-use (PFS) | 2 | - | - |
| Corticosteroid plus IM vs corticosteroids alone (OS) | 8 | 0.081 | 0.399 |
| Corticosteroid plus IM vs corticosteroids alone (PFS) | 8 | 0.805 | 0.905 |
| Corticosteroid plus IM vs corticosteroids alone (post-irAE OS) | 7 | 0.102 | 0.136 |
| Corticosteroid plus IM vs corticosteroids alone (post-irAE PFS) | 7 | 0.771 | 1.000 |
| Cumulative corticosteroid dose (post-irAE OS) | 7 | 0.325 | 0.562 |
| Cumulative corticosteroid dose (post-irAE PFS) | 7 | 0.022 | 0.239 |
Tab. 4
Results of sensitivity analysis"
| Risk factor | Fixed effect model | Random effect model |
|---|---|---|
| Corticosteroid use vs non-use (OS) | 0.73 (0.45-1.18) | 0.73 (0.04-14.55) |
| Corticosteroid use vs non-use (PFS) | 0.81 (0.57-1.15) | 0.68 (0.00-98.01) |
| Corticosteroid plus IM vs corticosteroids alone (OS) | 1.42 (1.09-1.85) | 1.66 (0.82-3.34) |
| Corticosteroid plus IM vs corticosteroids alone (PFS) | 1.21 (0.96-1.52) | 1.24 (0.80-1.91) |
| Corticosteroid plus IM vs corticosteroids alone (post-irAE OS) | 1.40 (1.11-1.76) | 1.50 (0.90-2.49) |
| Corticosteroid plus IM vs corticosteroids alone (post-irAE PFS) | 1.32 (1.08-1.62) | 1.32 (1.04-1.69) |
| Cumulative corticosteroid dose (post-irAE OS) | 0.95 (0.92-0.98) | 0.95 (0.91-0.98) |
| Cumulative corticosteroid dose (post-irAE PFS) | 0.96 (0.94-0.99) | 0.96 (0.92-1.00) |
Supplementary Ⅰ
Comprehensive forest plot data for all treatment combinations"
| Combination | Study | HR | 95% CI | Weight/% | Model |
|---|---|---|---|---|---|
| Corticosteroid use vs non-use (OS) | Robesti, et al | 0.86 | 0.48-1.54 | 67.62 | |
| Pichler, et al | 0.52 | 0.22-1.21 | 32.38 | ||
| Pooled effect | 0.73 | 0.45-1.18 | Fixed-effect | ||
| Corticosteroid use vs non-use (PFS) | Robesti, et al | 0.92 | 0.63-1.35 | 62.5 | |
| Pichler, et al | 0.41 | 0.17-0.99 | 37.5 | ||
| Pooled effect | 0.68 | 0.00-98.01 | Random-effect | ||
| Corticosteroid plus IM vs corticosteroids alone (post-irAE OS) | Verheijden, et al | 1.34 | 0.99-1.82 | 57.38 | |
| Verheijden, et al/CheckMate 067 | 0.93 | 0.50-1.72 | 14.07 | ||
| Verheijden, et al/CheckMate 142 | 6.10 | 0.27-137.42 | 0.55 | ||
| Verheijden, et al/CheckMate 214 | 1.41 | 0.68-2.92 | 10.02 | ||
| Verheijden, et al/CheckMate 648 | 1.49 | 0.44-5.08 | 3.53 | ||
| Verheijden, et al/CheckMate 743 | 1.46 | 0.71-3.01 | 10.15 | ||
| Verheijden, et al/CheckMate 9LA | 6.80 | 2.24-20.65 | 4.31 | ||
| Pooled effect | 1.40 | 1.11-1.76 | Fixed-effect | ||
| Corticosteroid plus IM vs corticosteroids alone (post-irAE PFS) | Verheijden, et al | 1.32 | 1.02-1.71 | 60.39 | |
| Verheijden, et al/CheckMate 067 | 1.31 | 0.70-2.45 | 10.51 | ||
| Verheijden, et al/CheckMate 142 | 1.17 | 0.15-9.11 | 0.98 | ||
| Verheijden, et al/CheckMate 214 | 1.04 | 0.59-1.82 | 13.12 | ||
| Verheijden, et al/CheckMate 648 | 0.73 | 0.22-2.44 | 2.84 | ||
| Verheijden, et al/CheckMate 743 | 1.60 | 0.80-3.20 | 8.54 | ||
| Verheijden, et al/CheckMate 9LA | 3.70 | 1.27-10.76 | 3.62 | ||
| Pooled effect | 1.32 | 1.08-1.62 | Fixed-effect | ||
| Corticosteroid plus IM vs corticosteroids alone (OS) | Curkovic, et al | 1.94 | 1.12-3.36 | 16.29 | |
| Syed, et al | 0.82 | 0.46-1.47 | 15.97 | ||
| Verheijden, et al/CheckMate 067 | 0.94 | 0.51-1.74 | 15.65 | ||
| Verheijden, et al/CheckMate 142 | 6.63 | 0.31-142.69 | 2.75 | ||
| Verheijden, et al/CheckMate 214 | 1.47 | 0.71-3.04 | 14.5 | ||
| Verheijden, et al/CheckMate 648 | 1.28 | 0.38-4.35 | 9.91 | ||
| Verheijden, et al/CheckMate 743 | 1.36 | 0.66-2.81 | 14.51 | ||
| Verheijden, et al/CheckMate 9LA | 12.19 | 3.82-38.88 | 10.42 | ||
| Pooled effect | 1.66 | 0.82-3.34 | Random-effect | ||
| Corticosteroid plus IM vs corticosteroids alone (PFS) | Syed, et al | 0.75 | 0.46-1.23 | 18.08 | |
| Curkovic, et al | 1.63 | 0.99-2.66 | 18.05 | ||
| Verheijden, et al/CheckMate 067 | 1.44 | 0.82-2.53 | 16.12 | ||
| Verheijden, et al/CheckMate 142 | 1.04 | 0.15-7.32 | 2.68 | ||
| Verheijden, et al/CheckMate 214 | 1.05 | 0.62-1.78 | 17.09 | ||
| Verheijden, et al/CheckMate 648 | 0.59 | 0.18-1.95 | 6.23 | ||
| Verheijden, et al/CheckMate 743 | 1.18 | 0.61-2.29 | 13.7 | ||
| Verheijden, et al/CheckMate 9LA | 4.46 | 1.63-12.22 | 8.05 | ||
| Pooled effect | 1.24 | 0.80-1.91 | Random-effect | ||
| Cumulative corticosteroid dose (post-irAE OS) | Verheijden, et al | 0.93 | 0.88-0.98 | 36.13 | |
| Verheijden, et al/CheckMate 067 | 0.97 | 0.91-1.04 | 23.47 | ||
| Verheijden, et al/CheckMate 142 | 8.96 | 0.45-179.27 | 0.01 | ||
| Verheijden, et al/CheckMate 214 | 0.98 | 0.91-1.06 | 17.98 | ||
| Verheijden, et al/CheckMate 648 | 0.98 | 0.85-1.13 | 5.16 | ||
| Verheijden, et al/CheckMate 743 | 0.90 | 0.82-0.98 | 13.47 | ||
| Verheijden, et al/CheckMate 9LA | 0.89 | 0.75-1.05 | 3.78 | ||
| Pooled effect | 0.95 | 0.92-0.98 | Fixed-effect | ||
| Cumulative corticosteroid dose (post-irAE PFS) | Verheijden, et al | 0.94 | 0.90-0.98 | 36.03 | |
| Verheijden, et al/CheckMate 067 | 0.96 | 0.90-1.03 | 14.35 | ||
| Verheijden, et al/CheckMate 142 | 1.37 | 1.06-1.78 | 0.97 | ||
| Verheijden, et al/CheckMate 214 | 0.99 | 0.94-1.05 | 21.33 | ||
| Verheijden, et al/CheckMate 648 | 0.98 | 0.87-1.10 | 4.75 | ||
| Verheijden, et al/CheckMate 743 | 0.94 | 0.87-1.02 | 10.33 | ||
| Verheijden, et al/CheckMate 9LA | 0.96 | 0.89-1.03 | 12.24 | ||
| Pooled effect | 0.96 | 0.94-0.99 | Fixed-effect |
| [1] |
RITTMEYER A, BARLESI F, WATERKAMP D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial[J]. Lancet, 2017, 389(10066): 255-265.
doi: S0140-6736(16)32517-X pmid: 27979383 |
| [2] |
HODI F S, O’DAY S J, MCDERMOTT D F, et al. Improved survival with ipilimumab in patients with metastatic melanoma[J]. N Engl J Med, 2010, 363(8): 711-723.
doi: 10.1056/NEJMoa1003466 |
| [3] |
ROBERT C, LONG G V, BRADY B, et al. Nivolumab in previously untreated melanoma withoutBRAF mutation[J]. N Engl J Med, 2015, 372(4): 320-330.
doi: 10.1056/NEJMoa1412082 |
| [4] |
JOHNSON D B, CHANDRA S, SOSMAN J A. Immune checkpoint inhibitor toxicity in 2018[J]. JAMA, 2018, 320(16): 1702-1703.
doi: 10.1001/jama.2018.13995 pmid: 30286224 |
| [5] |
NAIDOO J, WANG X, WOO K M, et al. Pneumonitis in patients treated with anti-programmed death-1/programmed death ligand 1 therapy[J]. J Clin Oncol, 2017, 35(7): 709-717.
doi: 10.1200/JCO.2016.68.2005 pmid: 27646942 |
| [6] |
NAIDOO J, MURPHY C, ATKINS M B, et al. Society for Immunotherapy of Cancer (SITC) consensus definitions for immune checkpoint inhibitor-associated immune-related adverse events (irAEs) terminology[J]. J Immunother Cancer, 2023, 11(3): e006398.
doi: 10.1136/jitc-2022-006398 |
| [7] |
TEULINGS H E, LIMPENS J, JANSEN S N, et al. Vitiligo-like depigmentation in patients with stage Ⅲ-Ⅳ melanoma receiving immunotherapy and its association with survival: a systematic review and meta-analysis[J]. J Clin Oncol, 2015, 33(7): 773-781.
doi: 10.1200/JCO.2014.57.4756 |
| [8] |
SHAFQAT H, GOURDIN T, SION A. Immune-related adverse events are linked with improved progression-free survival in patients receiving anti-PD-1/PD-L1 therapy[J]. Semin Oncol, 2018, 45(3): 156-163.
doi: S0093-7754(18)30009-5 pmid: 30348532 |
| [9] |
HORVAT T Z, ADEL N G, DANG T O, et al. Immune-related adverse events, need for systemic immunosuppression, and effects on survival and time to treatment failure in patients with melanoma treated with ipilimumab at memorial Sloan Kettering cancer center[J]. J Clin Oncol, 2015, 33(28): 3193-3198.
doi: 10.1200/JCO.2015.60.8448 pmid: 26282644 |
| [10] |
BAI X, HU J N, BETOF WARNER A, et al. Early use of high-dose glucocorticoid for the management of IrAE is associated with poorer survival in patients with advanced melanoma treated with anti-PD-1 monotherapy[J]. Clin Cancer Res, 2021, 27(21): 5993-6000.
doi: 10.1158/1078-0432.CCR-21-1283 |
| [11] |
VAN BUREN I, MADISON C, KOHN A, et al. Survival among veterans receiving steroids for immune-related adverse events after immune checkpoint inhibitor therapy[J]. JAMA Netw Open, 2023, 6(10): e2340695.
doi: 10.1001/jamanetworkopen.2023.40695 |
| [12] |
BYRON Y, YEGOROVA-LEE S, TIO M. Do corticosteroids affect immunotherapy efficacy in malignancy-a systematic review[J]. Cancer Med, 2024, 13(18): e70254.
doi: 10.1002/cam4.v13.18 |
| [13] |
JESSURUN C A C, HULSBERGEN A F C, DE WIT A E, et al. The combined use of steroids and immune checkpoint inhibitors in brain metastasis patients: a systematic review and meta-analysis[J]. Neuro Oncol, 2021, 23(8): 1261-1272.
doi: 10.1093/neuonc/noab046 |
| [14] |
PETRELLI F, SIGNORELLI D, GHIDINI M, et al. Association of steroids use with survival in patients treated with immune checkpoint inhibitors: a systematic review and meta-analysis[J]. Cancers, 2020, 12(3): 546.
doi: 10.3390/cancers12030546 |
| [15] |
DE LA BRUYÈRE C L, SOUQUET P J, DALLE S, et al. Investigating the impact of immune-related adverse events, glucocorticoid use and immunotherapy interruption on long-term survival outcomes[J]. Cancers, 2021, 13(10): 2365.
doi: 10.3390/cancers13102365 |
| [16] |
SHIMOMURA K, YAMAGUCHI T, OYA Y, et al. Impact of corticosteroids for IrAEs on the clinical outcome of immunotherapy in patients with NSCLC[J]. Anticancer Res, 2022, 42(12): 5961-5969.
doi: 10.21873/anticanres.16106 pmid: 36456164 |
| [17] |
MATSUKANE R, SUETSUGU K, HATA K, et al. Systematic surveillance of immune-related adverse events in clinical practice and impact of subsequent steroid medication on survival outcomes[J]. Int J Clin Oncol, 2023, 28(7): 860-871.
doi: 10.1007/s10147-023-02349-3 pmid: 37169946 |
| [18] |
CARIOU P L, POBEL C, MICHOT J M, et al. Impact of immunosuppressive agents on the management of immune-related adverse events of immune checkpoint blockers[J]. Eur J Cancer, 2024, 204: 114065.
doi: 10.1016/j.ejca.2024.114065 |
| [19] |
ROBESTI D, NOCERA L, BELLADELLI F, et al. The immune-related adverse events paradox in locally advanced or metastatic urothelial cancer after atezolizumab immunotherapy: analysis of individual patient data from IMvigor210 and IMvigor211 trials[J]. BJU Int, 2024, 133(2): 158-168.
doi: 10.1111/bju.v133.2 |
| [20] |
SYED S, HINES J, BACCILE R, et al. Studying outcomes after steroid-sparing immunosuppressive agent vs steroid-only treatment for immune-related adverse events in non-small-cell lung cancer (NSCLC) and melanoma: a retrospective case-control study[J]. Cancers, 2024, 16(10): 1892.
doi: 10.3390/cancers16101892 |
| [21] |
VERHEIJDEN R J, BURGERS F H, JANSSEN J C, et al. Corticosteroids and other immunosuppressants for immune-related adverse events and checkpoint inhibitor effectiveness in melanoma[J]. Eur J Cancer, 2024, 207: 114172.
doi: 10.1016/j.ejca.2024.114172 |
| [22] |
CURKOVIC N B, IRLMEIER R, BAI X, et al. Impact of steroid dose and timing on efficacy of combination PD-1/CTLA-4 blockade[J]. Oncoimmunology, 2025, 14(1): 2494433.
doi: 10.1080/2162402X.2025.2494433 |
| [23] |
PICHLER R, FRITZ J, MAIER S, et al. Target trial emulation to evaluate the effect of immune-related adverse events on outcomes in metastatic urothelial cancer[J]. Cancer Immunol Immunother, 2024, 74(1): 30.
doi: 10.1007/s00262-024-03871-7 pmid: 39708183 |
| [24] |
VERHEIJDEN R J, DE GROOT J S, FABRIEK B O, et al. Corticosteroids for immune-related adverse events and checkpoint inhibitor efficacy: analysis of six clinical trials[J]. J Clin Oncol, 2024, 42(31): 3713-3724.
doi: 10.1200/JCO.24.00191 pmid: 39110922 |
| [25] |
BIER J, STEIGER S M, REICHARDT H M, et al. Protection of antigen-primed effector T cells from glucocorticoid-induced apoptosis in cell culture and in a mouse model of multiple sclerosis[J]. Front Immunol, 2021, 12: 671258.
doi: 10.3389/fimmu.2021.671258 |
| [26] |
HEROLD M J, MCPHERSON K G, REICHARDT H M. Glucocorticoids in T cell apoptosis and function[J]. Cell Mol Life Sci, 2006, 63(1): 60-72.
doi: 10.1007/s00018-005-5390-y pmid: 16314919 |
| [27] |
BIANCHI M, MENG C, IVASHKIV L B. Inhibition of IL-2-induced jak-STAT signaling by glucocorticoids[J]. Proc Natl Acad Sci USA, 2000, 97(17): 9573-9578.
pmid: 10920190 |
| [28] |
ADORISIO S, CANNARILE L, DELFINO D V, et al. Glucocorticoid and PD-1 cross-talk: does the immune system become confused[J]. Cells, 2021, 10(9): 2333.
doi: 10.3390/cells10092333 |
| [29] |
MAEDA N, MARUHASHI T, SUGIURA D, et al. Glucocorticoids potentiate the inhibitory capacity of programmed cell death 1 by up-regulating its expression on T cells[J]. J Biol Chem, 2019, 294(52): 19896-19906.
doi: 10.1074/jbc.RA119.010379 pmid: 31723031 |
| [30] |
ZHANG A, FAN T, LIU Y X, et al. Regulatory T cells in immune checkpoint blockade antitumor therapy[J]. Mol Cancer, 2024, 23(1): 251.
doi: 10.1186/s12943-024-02156-y pmid: 39516941 |
| [31] |
GOODMAN R S, JOHNSON D B, BALKO J M. Corticosteroids and cancer immunotherapy[J]. Clin Cancer Res, 2023, 29(14): 2580-2587.
doi: 10.1158/1078-0432.CCR-22-3181 pmid: 36648402 |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
沪ICP备12009617
Powered by Beijing Magtech Co. Ltd
