Welcome to China Oncology,
China Oncology ›› 2025, Vol. 35 ›› Issue (8): 808-814.doi: 10.19401/j.cnki.1007-3639.2025.08.010
• Review • Previous Articles
WEN Yawen( ), SUN Li, ZHENG Xiangpeng(
), SUN Li, ZHENG Xiangpeng( )
)
Received:2025-03-13
Revised:2025-06-11
Online:2025-08-30
Published:2025-09-10
Contact:
ZHENG Xiangpeng
Supported by:Share article
CLC Number:
WEN Yawen, SUN Li, ZHENG Xiangpeng. Derepression of retrotransposable elements in the development of radiation-induced late effects: advancements and perspective[J]. China Oncology, 2025, 35(8): 808-814.
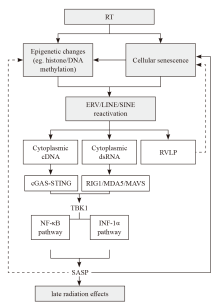
Fig. 1
The simplified conceptual diagram of radiation-induced reactivation of retrotransposable elements contributing to autoimmune inflammation-like late effects of radiotherapy Multiple downstream pathways may be involved including interferon and senescence-related signaling cascades as listed herein. RT: Radiotherapy; RVLP: Retrovirus-like particles; SASP: Senescence-associated secretory phenotype."
| [1] | ICRP A O B O, STEWART F A, AKLEYEV A V, et al. ICRP publication 118: ICRP statement on tissue reactions and early and late effects of radiation in normal tissues and organs: threshold doses for tissue reactions in a radiation protection context[J]. Ann ICRP, 2012, 41(1/2): 1-322. |
| [2] |
BENTZEN S M. Preventing or reducing late side effects of radiation therapy: radiobiology meets molecular pathology[J]. Nat Rev Cancer, 2006, 6(9): 702-713.
doi: 10.1038/nrc1950 pmid: 16929324 |
| [3] |
CARDELLI M. The epigenetic alterations of endogenous retroelements in aging[J]. Mech Ageing Dev, 2018, 174: 30-46.
doi: S0047-6374(17)30291-9 pmid: 29458070 |
| [4] |
JOHNSON W E. Endogenous retroviruses in the genomics era[J]. Annu Rev Virol, 2015, 2(1): 135-159.
doi: 10.1146/annurev-virology-100114-054945 pmid: 26958910 |
| [5] | DOPKINS N, NIXON D F. Activation of human endogenous retroviruses and its physiological consequences[J]. Nat Rev Mol Cell Biol, 2024, 25(3): 212-222. |
| [6] |
DOPKINS N, O’MARA M M, LAWRENCE E, et al. A field guide to endogenous retrovirus regulatory networks[J]. Mol Cell, 2022, 82(20): 3763-3768.
doi: 10.1016/j.molcel.2022.09.011 pmid: 36270247 |
| [7] | MERENCIANO M, LARUE A, GARAMBOIS C, et al. Exploring the relationship of transposable elements and ageing: causes and consequences[J]. Genome Biol Evol, 2025, 17(6): evaf088. |
| [8] | ZHAO X R, ZONG J B, LIU Y X, et al. Endogenous retroviruses unveiled: a comprehensive review of inflammatory signaling/senescence-related pathways and therapeutic strategies[J]. Aging Dis, 2024, 16(2): 738-756. |
| [9] |
WANG F, LI K Y, WANG W S, et al. Sensing of endogenous retroviruses-derived RNA by ZBP1 triggers PANoptosis in DNA damage and contributes to toxic side effects of chemotherapy[J]. Cell Death Dis, 2024, 15(10): 779.
doi: 10.1038/s41419-024-07175-7 pmid: 39465258 |
| [10] | SCHMIDLEITHNER L, STÜVE P, FEUERER M. Transposable elements as instructors of the immune system[J]. Nat Rev Immunol, 2025. |
| [11] | KASSIOTIS G. The immunological conundrum of endogenous retroelements[J]. Annu Rev Immunol, 2023, 41: 99-125. |
| [12] |
LIU X Q, LIU Z P, WU Z M, et al. Resurrection of endogenous retroviruses during aging reinforces senescence[J]. Cell, 2023, 186(2): 287-304.e26.
doi: 10.1016/j.cell.2022.12.017 pmid: 36610399 |
| [13] |
MIN X L, ZHENG M L, YU Y Q, et al. Ultraviolet light induces HERV expression to activate RIG-I signalling pathway in keratinocytes[J]. Exp Dermatol, 2022, 31(8): 1165-1176.
doi: 10.1111/exd.14568 pmid: 35332586 |
| [14] | WANG R C, LI H D, WU J F, et al. Gut stem cell necroptosis by genome instability triggers bowel inflammation[J]. Nature, 2020, 580(7803): 386-390. |
| [15] | MISHRA S, DEY A A, KESAVARDHANA S. Z-nucleic acid sensing and activation of ZBP1 in cellular physiology and disease pathogenesis[J]. Immunol Rev, 2025, 329(1): e13437. |
| [16] | LIU Y, MOLCHANOV V, ZHAO Y G, et al. H3K9me3 loss and ERVs activation as hallmarks for osteoarthritis progression and knee joint aging[J]. Osteoarthritis Cartilage, 2025, 33(1): 128-133. |
| [17] | CABRÉ N, FONDEVILA M F, WEI W C, et al. Activation of intestinal endogenous retroviruses by alcohol exacerbates liver disease[J]. J Clin Invest, 2025, 135(13): e188541. |
| [18] | MEEVASSANA J, SERIRODOM S, PRABSATTRU P, et al. Alu repetitive sequence CpG methylation changes in burn scars[J]. Burns, 2022, 48(6): 1417-1424. |
| [19] | SHANKARAPPA B, MAHADEVAN J, MURTHY P, et al. Hypomethylation of long interspersed nucleotide elements and aldehyde dehydrogenase in patients of alcohol use disorder with cirrhosis[J]. DNA Cell Biol, 2023, 42(7): 364-371. |
| [20] |
DHILLON P, MULHOLLAND K A, HU H L, et al. Increased levels of endogenous retroviruses trigger fibroinflammation and play a role in kidney disease development[J]. Nat Commun, 2023, 14(1): 559.
doi: 10.1038/s41467-023-36212-w pmid: 36732547 |
| [21] | RODRIGUEZ-SANABRIA J S, ROSAS-CAMPOS R, VÁZQUEZ-ESQUEDA Á, et al. H3K9me3 demethylation by JMJD2B is regulated by pirfenidone resulting in improved NASH[J]. Sci Rep, 2024, 14(1): 24714. |
| [22] |
SCHMIDT J, ERFLE V, MÜLLER W A. Activation of endogenous C-type retroviral genomes by internal alpha-irradiation of mice with 224Radium[J]. Radiat Environ Biophys, 1985, 24(1): 17-25.
pmid: 2983362 |
| [23] | The 2007 recommendations of the international commission on radiological protection. ICRP publication 103[J]. Ann ICRP, 2007, 37(2/3/4): 1-332. |
| [24] | LEE H G, RONE J M, LI Z R, et al. Disease-associated astrocyte epigenetic memory promotes CNS pathology[J]. bioRxiv, 2024: 2024.01.04.574196. |
| [25] | BARNES B M, SHYNE A, GUNN D A, et al. Epigenetics and ultraviolet radiation: implications for skin ageing and carcinogenesis[J]. Skin Health Dis, 2024, 4(6): e410. |
| [26] |
BIAN X W, PIIPPONEN M, LIU Z, et al. Epigenetic memory of radiotherapy in dermal fibroblasts impairs wound repair capacity in cancer survivors[J]. Nat Commun, 2024, 15(1): 9286.
doi: 10.1038/s41467-024-53295-1 pmid: 39468077 |
| [27] |
PARK J, LEE H J, HAN Y K, et al. Identification of DNA methylation biomarkers for evaluating cardiovascular disease risk from epigenome profiles altered by low-dose ionizing radiation[J]. Clin Epigenetics, 2024, 16(1): 19.
doi: 10.1186/s13148-024-01630-0 pmid: 38303056 |
| [28] | AHMAD CHAUDHRY M, OMARUDDIN R A. Differential DNA methylation alterations in radiation-sensitive and-resistant cells[J]. DNA Cell Biol, 2012, 31(6): 908-916. |
| [29] | VARSHNEY D, VAVROVA-ANDERSON J, OLER A J, et al. SINE transcription by RNA polymerase Ⅲ is suppressed by histone methylation but not by DNA methylation[J]. Nat Commun, 2015, 6: 6569. |
| [30] |
BROCKS D, SCHMIDT C R, DASKALAKIS M, et al. DNMT and HDAC inhibitors induce cryptic transcription start sites encoded in long terminal repeats[J]. Nat Genet, 2017, 49(7): 1052-1060.
doi: 10.1038/ng.3889 pmid: 28604729 |
| [31] |
MAGER D L, LORINCZ M C. Epigenetic modifier drugs trigger widespread transcription of endogenous retroviruses[J]. Nat Genet, 2017, 49(7): 974-975.
doi: 10.1038/ng.3902 pmid: 28656984 |
| [32] | YAO Y, CHEN L F, LI J, et al. Altered DNA methylation and gene expression profiles in radiation-induced heart fibrosis of sprague-dawley rats[J]. Radiat Res, 2022, 198(2): 154-161. |
| [33] |
QIU Y Y, GAO Y Y, YU D J, et al. Genome-wide analysis reveals zinc transporter ZIP9 regulated by DNA methylation promotes radiation-induced skin fibrosis via the TGF-β signaling pathway[J]. J Invest Dermatol, 2020, 140(1): 94-102.e7.
doi: S0022-202X(19)31795-6 pmid: 31254515 |
| [34] |
BECKER B V, KAATSCH L, OBERMAIR R, et al. X-ray irradiation induces subtle changes in the genome-wide distribution of DNA hydroxymethylation with opposing trends in genic and intergenic regions[J]. Epigenetics, 2019, 14(1): 81-93.
doi: 10.1080/15592294.2019.1568807 pmid: 30691379 |
| [35] |
TERRAZZINO S, DEANTONIO L, CARGNIN S, et al. DNA methyltransferase gene polymorphisms for prediction of radiation-induced skin fibrosis after treatment of breast cancer: a multifactorial genetic approach[J]. Cancer Res Treat, 2017, 49(2): 464-472.
doi: 10.4143/crt.2016.256 pmid: 27554481 |
| [36] |
ISHAK C A, MARSHALL A E, PASSOS D T, et al. An RB-EZH2 complex mediates silencing of repetitive DNA sequences[J]. Mol Cell, 2016, 64(6): 1074-1087.
doi: S1097-2765(16)30666-9 pmid: 27889452 |
| [37] | ZHANG Y Y, YU C, AGBORBESONG E, et al. Downregulation of EZH2 promotes renal epithelial cellular senescence and kidney aging[J]. FASEB J, 2025, 39(9): e70605. |
| [38] |
SHAO Z Y, LU J W, KHUDAVERDYAN N, et al. Multi-layered heterochromatin interaction as a switch for DIM2-mediated DNA methylation[J]. Nat Commun, 2024, 15(1): 6815.
doi: 10.1038/s41467-024-51246-4 pmid: 39122718 |
| [39] | GEHRS S, GU Z G, HEY J, et al. DNMT3A-dependent DNA methylation shapes the endothelial enhancer landscape[J]. Nucleic Acids Res, 2025, 53(10): gkaf435. |
| [40] |
CAMERON E E, BACHMAN K E, MYÖHÄNEN S, et al. Synergy of demethylation and histone deacetylase inhibition in the re-expression of genes silenced in cancer[J]. Nat Genet, 1999, 21(1): 103-107.
pmid: 9916800 |
| [41] |
HUANG W J, HICKSON L J, EIRIN A, et al. Cellular senescence: the good, the bad and the unknown[J]. Nat Rev Nephrol, 2022, 18(10): 611-627.
doi: 10.1038/s41581-022-00601-z pmid: 35922662 |
| [42] | SURYADEVARA V, HUDGINS A D, RAJESH A, et al. SenNet recommendations for detecting senescent cells in different tissues[J]. Nat Rev Mol Cell Biol, 2024, 25(12): 1001-1023. |
| [43] |
KIM J H, BROWN S L, GORDON M N. Radiation-induced senescence: therapeutic opportunities[J]. Radiat Oncol, 2023, 18(1): 10.
doi: 10.1186/s13014-022-02184-2 pmid: 36639774 |
| [44] | BLOKLAND K E C, WATERS D W, SCHULIGA M, et al. Senescence of IPF lung fibroblasts disrupt alveolar epithelial cell proliferation and promote migration in wound healing[J]. Pharmaceutics, 2020, 12(4): 389. |
| [45] | RYAN P, LEE J. In vitro senescence and senolytic functional assays[J]. Biomater Sci, 2025, 13(13): 3509-3531. |
| [46] | ZHU J J, AO X K, LIU Y H, et al. TNKS1BP1 mediates AEC Ⅱ senescence and radiation induced lung injury through suppressing EEF2 degradation[J]. Respir Res, 2024, 25(1): 299. |
| [47] | DE CECCO M, ITO T, PETRASHEN A P, et al. L1 drives IFN in senescent cells and promotes age-associated inflammation[J]. Nature, 2019, 566(7742): 73-78. |
| [48] |
VAN METER M, KASHYAP M, REZAZADEH S, et al. SIRT6 represses LINE1 retrotransposons by ribosylating KAP1 but this repression fails with stress and age[J]. Nat Commun, 2014, 5: 5011.
doi: 10.1038/ncomms6011 pmid: 25247314 |
| [49] |
MAO J, ZHANG Q, ZHUANG Y, et al. Reactivation of senescence-associated endogenous retroviruses by ATF3 drives interferon signaling in aging[J]. Nat Aging, 2024, 4(12): 1794-1812.
doi: 10.1038/s43587-024-00745-6 pmid: 39543280 |
| [50] |
KREILING J A. Dysregulation of endogenous retroviruses triggers aging and senescence[J]. Nat Aging, 2024, 4(12): 1670-1672.
doi: 10.1038/s43587-024-00759-0 pmid: 39567758 |
| [51] |
YAHYAPOUR R, AMINI P, REZAPOUR S, et al. Radiation-induced inflammation and autoimmune diseases[J]. Mil Med Res, 2018, 5(1): 9.
doi: 10.1186/s40779-018-0156-7 pmid: 29554942 |
| [52] |
VIRET C, BYNOE M S. Human endogenous retroviruses expression in autoimmunity[J]. Yale J Biol Med, 2024, 97(4): 521-528.
doi: 10.59249/OIKF8301 pmid: 39703611 |
| [53] |
PEZONE A, OLIVIERI F, NAPOLI M V, et al. Inflammation and DNA damage: cause, effect or both[J]. Nat Rev Rheumatol, 2023, 19(4): 200-211.
doi: 10.1038/s41584-022-00905-1 pmid: 36750681 |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
沪ICP备12009617
Powered by Beijing Magtech Co. Ltd
