Welcome to China Oncology,
China Oncology ›› 2025, Vol. 35 ›› Issue (7): 685-694.doi: 10.19401/j.cnki.1007-3639.2025.07.007
• Article • Previous Articles Next Articles
LIU Dan( ), ZHANG Guxiang, XIE Dan, XU Yan, XIE Chengfang, TANG Yuxi
), ZHANG Guxiang, XIE Dan, XU Yan, XIE Chengfang, TANG Yuxi
Received:2024-10-23
Revised:2025-02-06
Online:2025-07-30
Published:2025-08-13
Contact:
ZHANG Guxiang
Supported by:Share article
CLC Number:
LIU Dan, ZHANG Guxiang, XIE Dan, XU Yan, XIE Chengfang, TANG Yuxi. Mechanism of immune escape mediated by T cell depletion induced by TOX signaling pathway in cervical cancer microenvironment[J]. China Oncology, 2025, 35(7): 685-694.
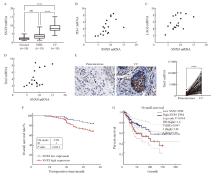
Fig. 1
The expression of snx9 is up-regulated in CC, which is related to poor prognosis A: The level of SNX9 in PBMC of normal cervix (n=18), high squamous intraepithelial lesions (n=18, HSIL) and CC patients (n=18). B-D: Correlation analysis of expressions of SNX9 and PD-1, SNX9 and LAG3, SNX9 and Tim3 in CD8+ T cells of PBMC in patients with CC. E: Comparison of SNX9+ cells in CC and adjacent tissues (DAB color, magnification 200 times). F: Kaplan-Meier was used to analyze the relationship between the expression of SNX9 in tumor tissues and the overall survival in CC patients (n=153). G: GEPIA online database was used to analyze the relationship between the expression of SNX9 in CC tissues and the overall survival time. NS: No significance; ****: P<0.000 1."
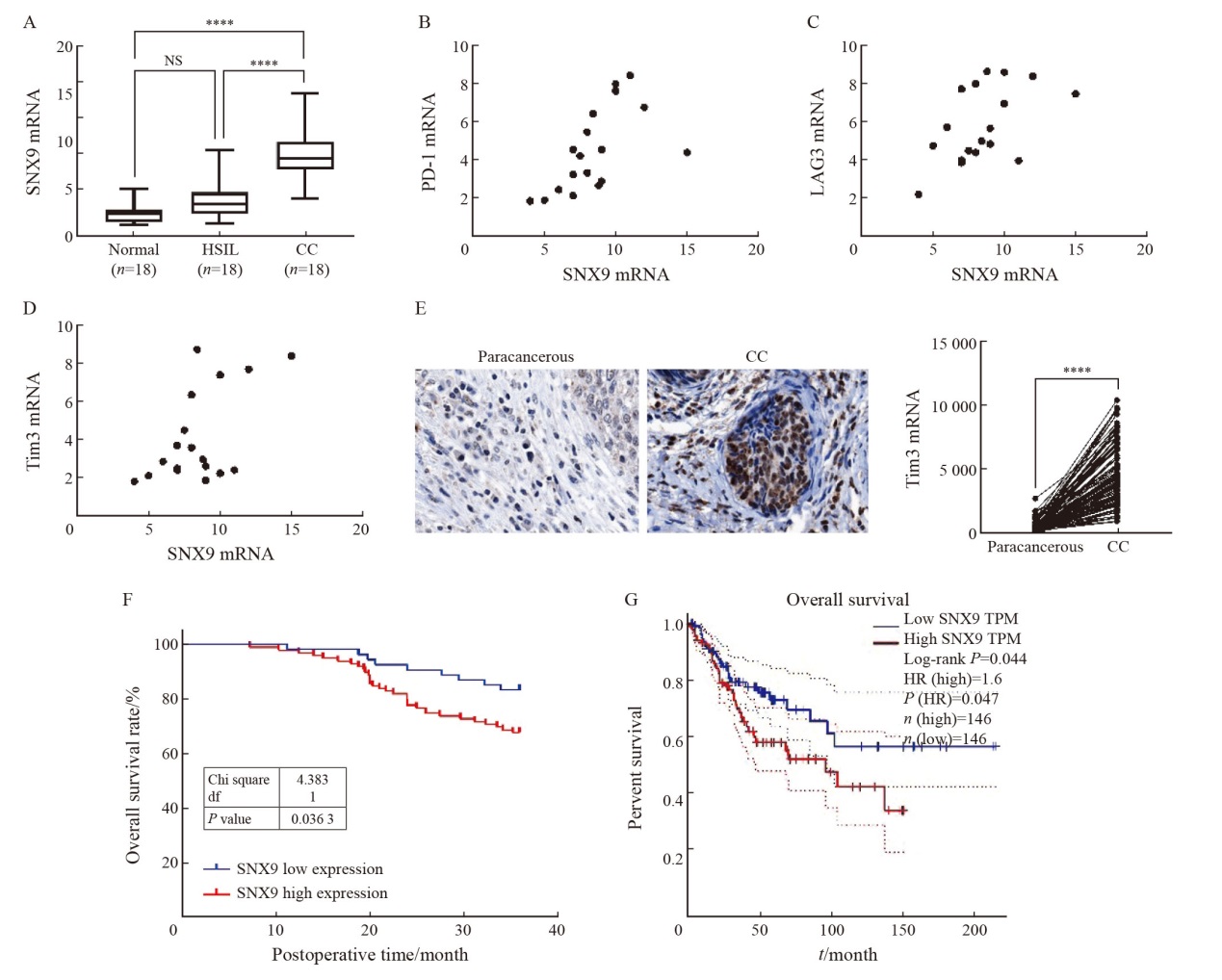
Tab. 1
Correlation between the expression of SNX9 in CC and clinicopathological factors [n (%)]"
| Characteristic | SNX9 expression | χ2 | P value | |
|---|---|---|---|---|
| Low (n=54) | High (n=99) | |||
| Age/year | 0.06 | 0.814 | ||
| <40 | 9 (16.7) | 18 (18.2) | ||
| >40 | 45 (83.3) | 81 (81.8) | ||
| Differentiation | 8.395 | 0.004 | ||
| High/moderate | 51 (94.4) | 75 (75.7) | ||
| Low/Poor | 3 (5.6) | 24 (24.3) | ||
| FIGO | 28.977 | <0.001 | ||
| Ⅰ | 51 (94.4) | 51 (51.5) | ||
| Ⅱ | 3 (5.6) | 48 (48.5) | ||
| Histology | 9.264 | 0.010 | ||
| Squamous cell carcinoma | 42 (77.7) | 87 (87.9) | ||
| Adenocarcinoma | 9 (16.7) | 3 (3.0) | ||
| Adenosquamous carcinoma | 3 (5.6) | 9 (9.1) | ||
| Tumor size/cm | 4.874 | 0.027 | ||
| ≤4 | 45 (83.3) | 66 (66.7) | ||
| >4 | 9 (16.7) | 33 (33.3) | ||
| Infiltration of uterine muscle layer | 0.618 | 0.432 | ||
| No | 18 (33.3) | 27 (27.3) | ||
| Yes | 36 (66.7) | 72 (72.7) | ||
| Parauterine infiltration | 30.884 | <0.001 | ||
| No | 54 (100) | 78 (78.8) | ||
| Yes | 0 (0.0) | 21 (21.2) | ||
| Vaginal infiltration | 17.461 | <0.001 | ||
| No | 51 (94.4) | 63 (63.6) | ||
| Yes | 3 (5.6) | 36 (36.4) | ||
| Pelvic lymph node metastasis | 35.875 | <0.001 | ||
| No | 51 (94.4) | 45 (45.5) | ||
| Yes | 3 (5.6) | 54 (54.5) | ||

Fig. 2
CD8+SNX9+ T cells were highly expressed in PBMC of CC patients A: CD8+SNX9+ T cells in PBMC of normal cervix (n=18), HSIL(n=18) and CC patients (n=18). B-C: CD8+ T cells were isolated from PBMC. The levels of TNF-α and IFN-γ secreted by CD8+SNX9+T cells and CD8+SNX9-T cells were detected by ELISpot. *: P<0.05; ***: P<0.001; ****: P <0.000 1."
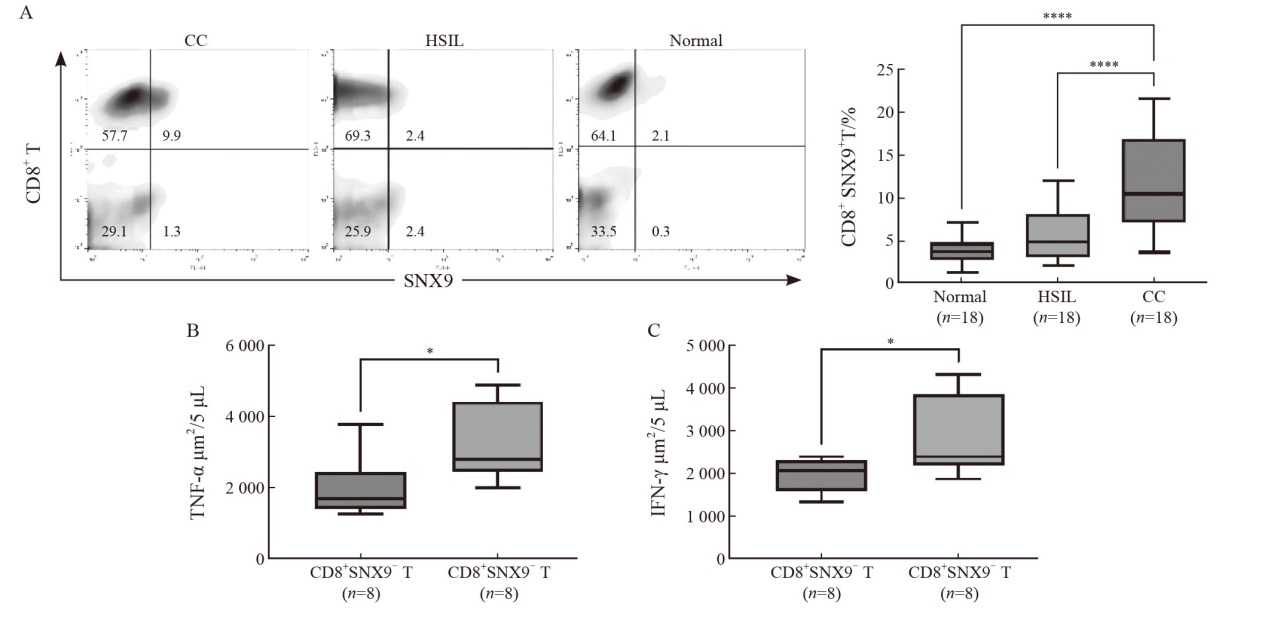
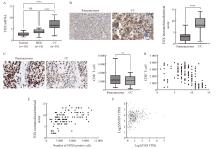
Fig. 3
Up-regulation of SNX9/TOX expression in CC tissue is related to CD8+ T cell depletion A: TOX mRNA levels in normal cervix (n=18), HSIL(n=18) and CC patients (n=18) PBMC. B, C: Comparison of TOX immunohistochemical score and CD8+ T cell level in CC and adjacent tissues (DAB staining, magnification 200 times). D: Correlation analysis between TOX immunohistochemical score and CD8+ T cell level in CC tissues. E: Correlation analysis between TOX immunohistochemical score and SNX9 positive cell level in CC tissues. F: GEPIA online database was used to analyze the correlation between SNX9 and TOX expression in CC tissues. NS: No significance; **: P<0.01; ****: P<0.000 1."
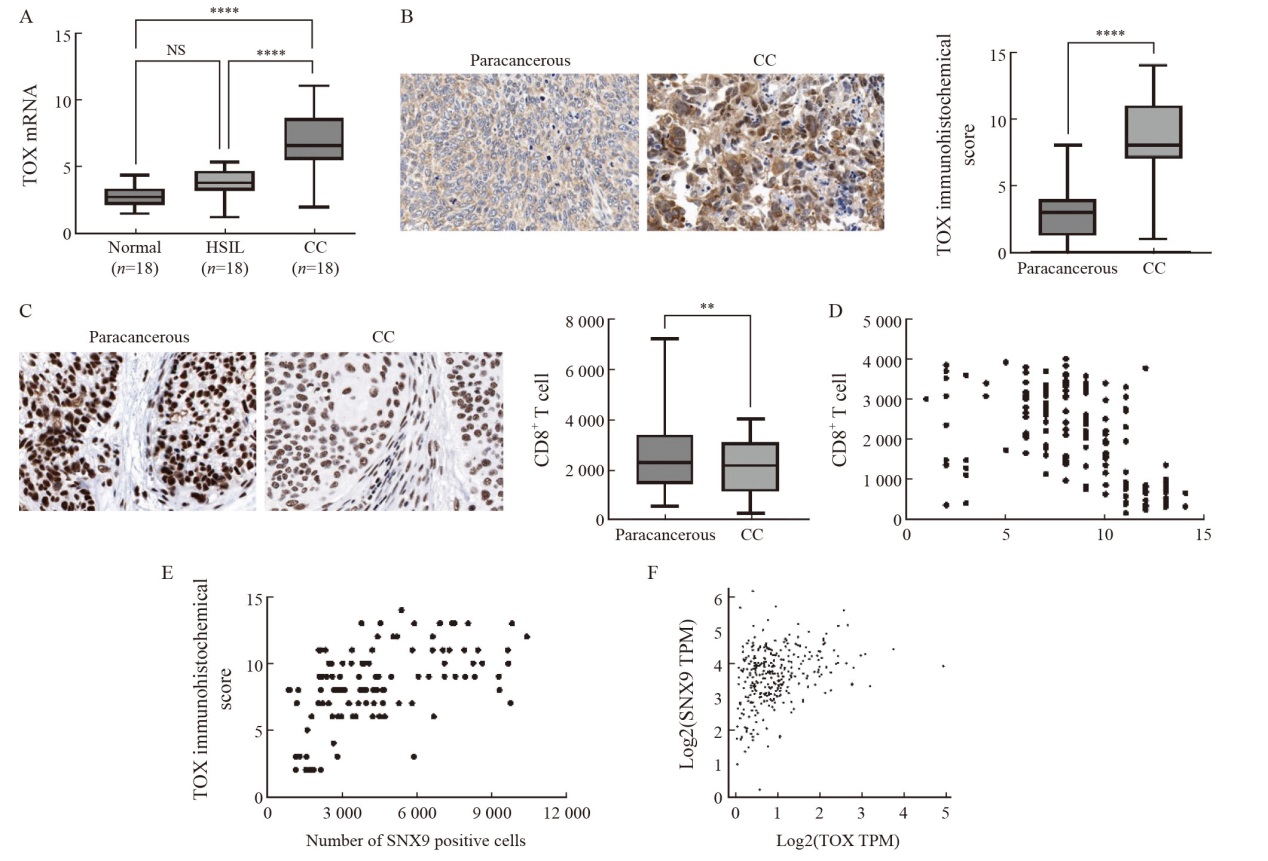
Fig. 4
SNX9 and PD-1 inhibit the expression of p-IκBα and p-P65 proteins and CD8+ T cells produce cytokines A: Immunoblot analysis of SNX9, TOX, p-IκBα and p-P65 protein expression in CD8+ T cells. B, C: ELISpot were used to detect the ability of CD8+ T cells to secrete cytokines IFN-γ and TNF-α. Stimulate CD8+ T cells with αCD3/CD28 and co-culture with tumor cells in the presence of anti-SNX9 antibodies, anti-PD-1 blocking antibodies, or isotype controls. D: Immunoblot analysis of SNX9, TOX, p-IκBα and p-P65 protein expression in CD8+ T cells. E, F: ELISpot were used to detect the ability of CD8+ T cells to secrete cytokines IFN-γ and TNF-α. *: P<0.05; **: P<0.01; ***: P <0.001; ****: P<0.000 1."
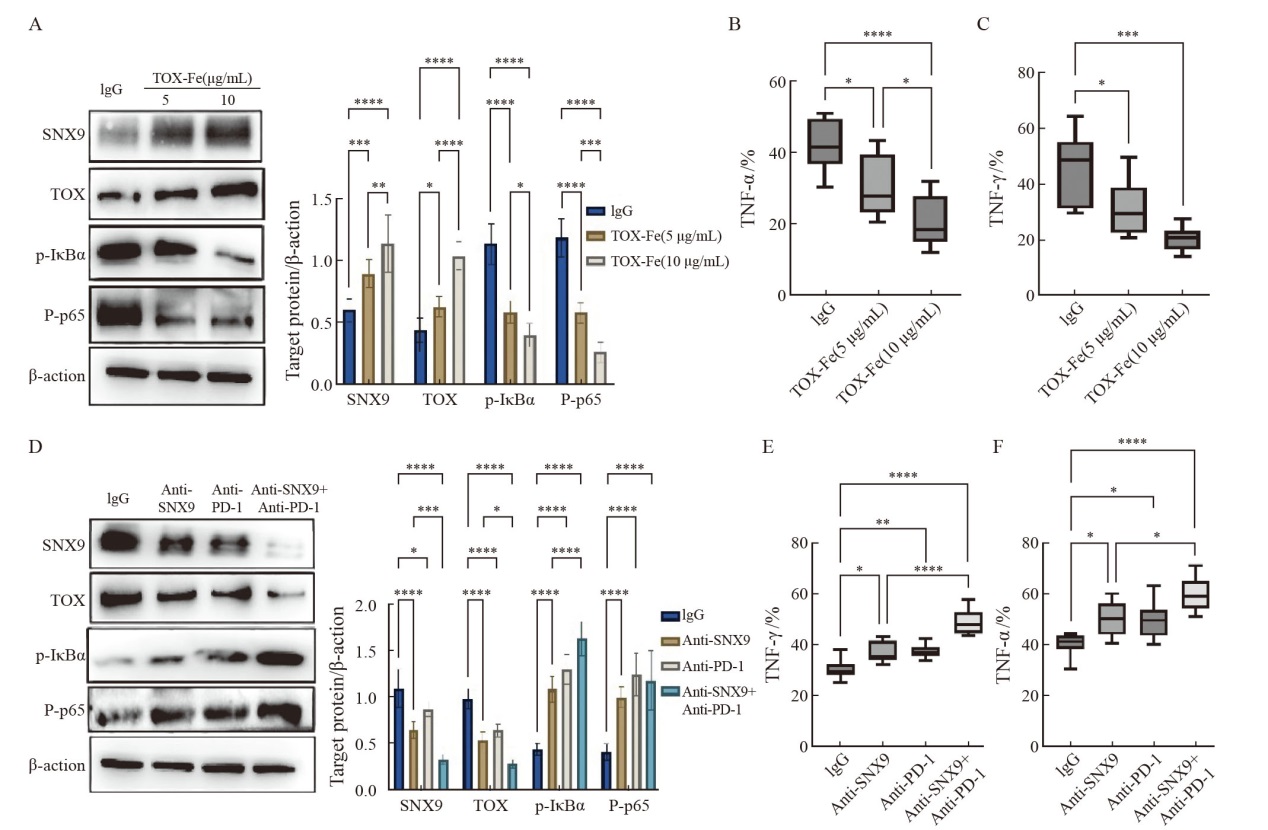
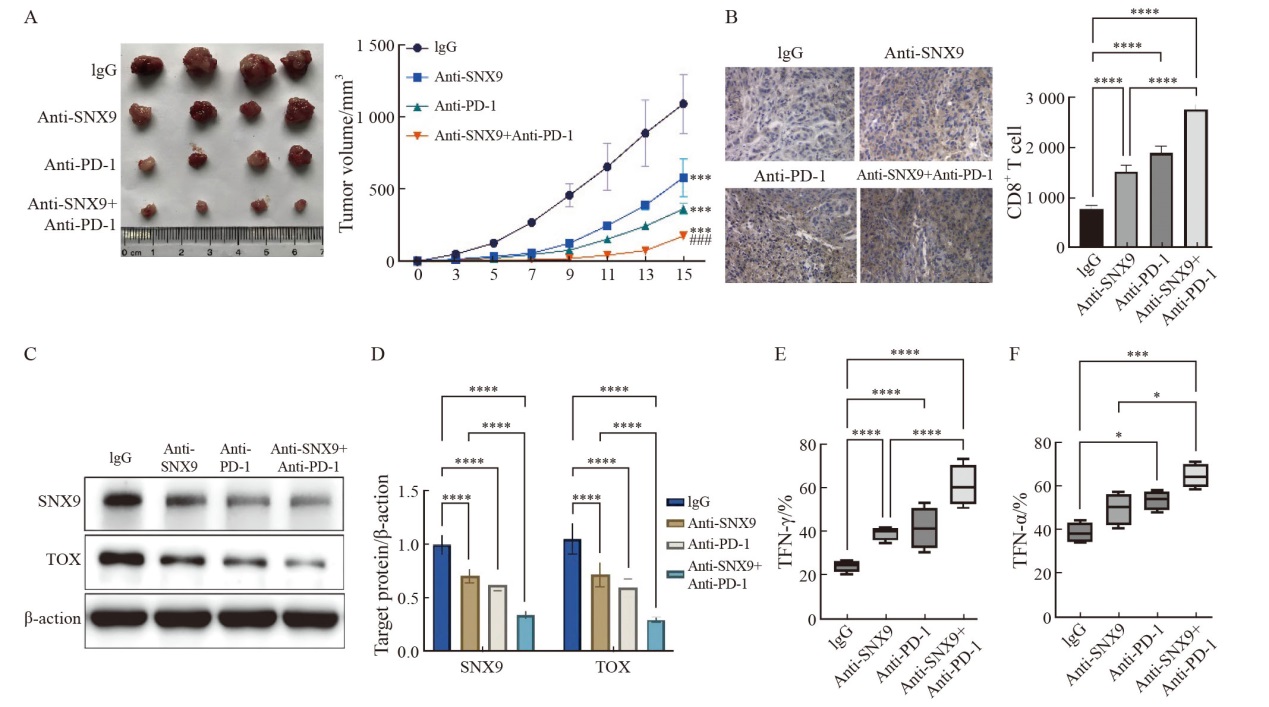
Fig.5
Effects of SNX9 inhibition on the growth of transplanted cervical tumor and the function of CD8+ T lymphocytes in C57BL/6 mice A: Analysis of tumor progression in WT-U14 cell transplantation mice treated with PD-1 blocking antibody, SNX9 blocking antibody, or isotype control. B: Immunohistochemical analysis of CD8+ T cell infiltration levels in mouse tumor tissues (DAB staining, magnification 200×). C: Immunoblot analysis of SNX9 and TOX protein expression in tumor tissues. D, E: ELISpot were used to detect the ability of CD8+ T cells in mice receiving different treatments to secrete TNF-α and IFN-γ. *: P<0.05; **: P<0.01; ***: P<0.001; ****: P<0.000 1."
| [1] | HUANG J J, DENG Y Y, BOAKYE D, et al. Global distribution, risk factors, and recent trends for cervical cancer: a worldwide country-level analysis[J]. Gynecol Oncol, 2022, 164(1): 85-92. |
| [2] | SINGH D, VIGNAT J, LORENZONI V, et al. Global estimates of incidence and mortality of cervical cancer in 2020: a baseline analysis of the WHO Global Cervical Cancer Elimination Initiative[J]. Lancet Glob Health, 2023, 11(2): e197-e206. |
| [3] | LIU L, WANG A H, LIU X L, et al. Blocking TIGIT/CD155 signalling reverses CD8+ T cell exhaustion and enhances the antitumor activity in cervical cancer[J]. J Transl Med, 2022, 20(1): 280. |
| [4] |
SHARMA P, GOSWAMI S, RAYCHAUDHURI D, et al. Immune checkpoint therapy-current perspectives and future directions[J]. Cell, 2023, 186(8): 1652-1669.
doi: 10.1016/j.cell.2023.03.006 pmid: 37059068 |
| [5] |
LORUSSO D, XIANG Y, HASEGAWA K, et al. Pembrolizumab or placebo with chemoradiotherapy followed by pembrolizumab or placebo for newly diagnosed, high-risk, locally advanced cervical cancer (ENGOT-cx11/GOG-3047/KEYNOTE-A18): a randomised, double-blind, phase 3 clinical trial[J]. Lancet, 2024, 403(10434): 1341-1350.
doi: 10.1016/S0140-6736(24)00317-9 pmid: 38521086 |
| [6] |
OAKNIN A, MOORE K, MEYER T, et al. Nivolumab with or without ipilimumab in patients with recurrent or metastatic cervical cancer (CheckMate 358): a phase 1-2, open-label, multicohort trial[J]. Lancet Oncol, 2024, 25(5): 588-602.
doi: 10.1016/S1470-2045(24)00088-3 pmid: 38608691 |
| [7] |
TREFNY M P, KIRCHHAMMER N, DER MAUR P A, et al. Deletion of SNX9 alleviates CD8 T cell exhaustion for effective cellular cancer immunotherapy[J]. Nat Commun, 2023, 14(1): 86.
doi: 10.1038/s41467-022-35583-w pmid: 36732507 |
| [8] | PHILIP M, SCHIETINGER A. CD8+ T cell differentiation and dysfunction in cancer[J]. Nat Rev Immunol, 2022, 22(4): 209-223. |
| [9] | CHEN J, SONG Y, MIAO F, et al. PDL1-positive exosomes suppress antitumor immunity by inducing tumor-specific CD8+ T cell exhaustion during metastasis[J]. Cancer Sci, 2021, 112(9): 3437-3454. |
| [10] |
BAI R L, LV Z, XU D S, et al. Predictive biomarkers for cancer immunotherapy with immune checkpoint inhibitors[J]. Biomark Res, 2020, 8: 34.
doi: 10.1186/s40364-020-00209-0 pmid: 32864131 |
| [11] | MAKUKU R, KHALILI N, RAZI S, et al. Current and future perspectives of PD-1/PDL-1 blockade in cancer immunotherapy[J]. J Immunol Res, 2021, 2021: 6661406. |
| [12] | WANG Y Z, WANG J S, DU J, et al. Clinical benefit analysis of PD-1 inhibitors in patients with advanced, recurrent or metastatic cervical cancer: a meta-analysis and systematic review[J]. Front Immunol, 2024, 15: 1305810. |
| [13] | LEE J B, KHAN D H, HURREN R, et al. Venetoclax enhances T cell-mediated antileukemic activity by increasing ROS production[J]. Blood, 2021, 138(3): 234-245. |
| [14] | WEI W, CHEN Z N, WANG K. CRISPR/Cas9: a powerful strategy to improve CAR-T cell persistence[J]. Int J Mol Sci, 2023, 24(15): 12317. |
| [15] | NIU H Y, WANG H Q. TOX regulates T lymphocytes differentiation and its function in tumor[J]. Front Immunol, 2023, 14: 990419. |
| [16] | AHMAD A, ANSARI I A. Carvacrol exhibits chemopreventive potential against cervical cancer cells via caspase-dependent apoptosis and abrogation of cell cycle progression[J]. Anticancer Agents Med Chem, 2021, 21(16): 2224-2235. |
| [17] |
FENG X, SHAN R, HU X M. The linkage of NF-κB signaling pathway-associated long non-coding RNAs with tumor microenvironment and prognosis in cervical cancer[J]. BMC Med Genomics, 2023, 16(1): 169.
doi: 10.1186/s12920-023-01605-9 pmid: 37461017 |
| [18] | WANG D J, ZOU F, LI Y, et al. Targeting MELK improves PD-1 blockade efficiency in cervical cancer via enhancing antitumor immunity[J]. Mol Ther Oncol, 2024, 32(1): 200759. |
| [19] |
BORST J, BUSSELAAR J, BOSMA D M T, et al. Mechanism of action of PD-1 receptor/ligand targeted cancer immunotherapy[J]. Eur J Immunol, 2021, 51(8): 1911-1920.
doi: 10.1002/eji.202048994 pmid: 34106465 |
| [20] | BUDIMIR N, THOMAS G D, DOLINA J S, et al. Reversing T-cell exhaustion in cancer: lessons learned from PD-1/PD-L1 immune checkpoint blockade[J]. Cancer Immunol Res, 2022, 10(2): 146-153. |
| [21] | CILLO A R, CARDELLO C, SHAN F, et al. Blockade of LAG-3 and PD-1 leads to co-expression of cytotoxic and exhaustion gene modules in CD8+ T cells to promote antitumor immunity[J]. Cell, 2024, 187(16): 4373-4388.e15. |
| [22] | ECKER M, SCHREGLE R, KAPOOR-KAUSHIK N, et al. SNX9-induced membrane tubulation regulates CD28 cluster stability and signalling[J]. eLife, 2022, 11: e67550. |
| [1] | FAN Sumei, XIN Congling, ZHU Laifang, LIU Chang, XU Rui, ZHOU Zhengrong, CHENG Xi. Effectiveness and safety analysis of camrelizumab combined with chemotherapy and targeted therapy in patients with recurrent, metastatic, and treatment-naive advanced cervical cancer: a retrospective cohort study [J]. China Oncology, 2025, 35(6): 570-577. |
| [2] | CHEN Xun, ZHENG Zhenxia, RUAN Xueru. Effects of TMCO1 on proliferation and migration of cervical cancer cells [J]. China Oncology, 2024, 34(6): 571-580. |
| [3] | FENG Zheng, GUO Qinhao, ZHU Jun, WU Xiaohua, WEN Hao. Progress in treatment of gynecological cancer in 2023 [J]. China Oncology, 2024, 34(4): 340-360. |
| [4] | SHEN Jie, FENG Xiaoshuang, WEN Hao, ZHOU Changming, MO Miao, WANG Zezhou, YUAN Jing, WU Xiaohua, ZHENG Ying. Metastasis patterns and survival analysis of 572 patients with metastatic cervical cancer: a hospital-based real world study [J]. China Oncology, 2024, 34(4): 361-367. |
| [5] | XIA Lingfang, ZHU Jun, WU Xiaohua. The latest progress and prospect of gynecological tumor treatment at 2023 ESMO [J]. China Oncology, 2023, 33(11): 969-980. |
| [6] | GUO Qinhao, YU Min, WU Xiaohua. Progress in diagnosis and treatment of gynecological tumors in 2022 [J]. China Oncology, 2023, 33(1): 14-24. |
| [7] | PANG Yi, WU Chunxiao, GU Kai, BAO Pingping, WANG Chunfang, SHI Liang, GONG Yangming, XIANG Yongmei, DOU Jianming, WU Mengyin, FU Chen, SHI Yan. Analysis of current status of cervical cancer incidence and mortality in Shanghai, 2016 and trends of 2002-2016 [J]. China Oncology, 2022, 32(6): 519-526. |
| [8] | WANG Chuntao, GE Anxing, WU Hongyan, ZHANG Xueyan, YANG Sheng, YUAN Hongxiang, CHENG Yanping, FENG Yanlu, LU Xinyuan, LIANG Geyu. The association between cervical lesions of different grades and lncRNA HOTTIP and H19 single nucleotide polymorphisms [J]. China Oncology, 2022, 32(4): 324-334. |
| [9] | FANG Yanhui , MA Caijuan , ZHANG Chunli , ZHAI Jiawei , ZHU Qiaoying , LIU Yin , ZHAO Xiwa . miR-496 inhibits proliferation, migration and invasion of HeLa cell in cervical cancer via SFMBT1 [J]. China Oncology, 2021, 31(8): 697-703. |
| [10] | LONG Xingtao , ZHOU Qi , WANG Dong , CHEN Yuemei , JIN Fujun . The prognostic value of revised 2018 FIGO stage ⅢC in cervical cancer [J]. China Oncology, 2021, 31(8): 725-733. |
| [11] | DONG Shijie, HU Xiaoxin, WANG Wei, YANG Meng, YUE Lei, TONG Tong, GU Yajia. Prediction of lymph node metastasis of cervical cancer based on multi-sequence MRI and multi-system imaging omics model [J]. China Oncology, 2021, 31(6): 460-467. |
| [12] | ZHU Jun, WU Xiaohua. Leading research progress and prospect of gynecological oncology in 2020 [J]. China Oncology, 2021, 31(4): 250-256. |
| [13] | SHEN Jie , MO Miao , YUAN Jing , ZHOU Changming , WANG Zezhou , ZHANG Zhihong , WEN Hao , WU Xiaohua , ZHENG Ying . Long-term survival report on 1 313 patients with locally advanced cervical cancer: a hospital-based real world study [J]. China Oncology, 2020, 30(3): 192-198. |
| [14] | CHEN Xiaohui, WANG Jiazhou, HU Weigang, PENG Jiayuan, ZHAI Peng. Dosimetric impact of machine and treatment planning system on knowledge-based planning: a study based on cervical cancer IMRT plan [J]. China Oncology, 2020, 30(10): 821-825. |
| [15] | WANG Li,REN Yulan,WANG Huaying . Pulmonary embolism after radical surgery of cervical cancer: a case report and the review of literature [J]. China Oncology, 2019, 29(8): 599-601. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
沪ICP备12009617
Powered by Beijing Magtech Co. Ltd
