Welcome to China Oncology,
China Oncology ›› 2024, Vol. 34 ›› Issue (7): 669-679.doi: 10.19401/j.cnki.1007-3639.2024.07.006
• Article • Previous Articles Next Articles
LIAO Ziyi( ), PENG Yang, ZENG Beilei, MA Yingying, ZENG Li, GAN Kelun, MA Daiyuan(
), PENG Yang, ZENG Beilei, MA Yingying, ZENG Li, GAN Kelun, MA Daiyuan( )
)
Received:2023-11-06
Revised:2024-04-25
Online:2024-07-30
Published:2024-08-08
Contact:
MA Daiyuan
Share article
CLC Number:
LIAO Ziyi, PENG Yang, ZENG Beilei, MA Yingying, ZENG Li, GAN Kelun, MA Daiyuan. Analysis of pathological remission degree and influencing factors of radical surgery after neoadjuvant immunotherapy combined with chemotherapy in patients with locally advanced esophageal squamous cell carcinoma[J]. China Oncology, 2024, 34(7): 669-679.

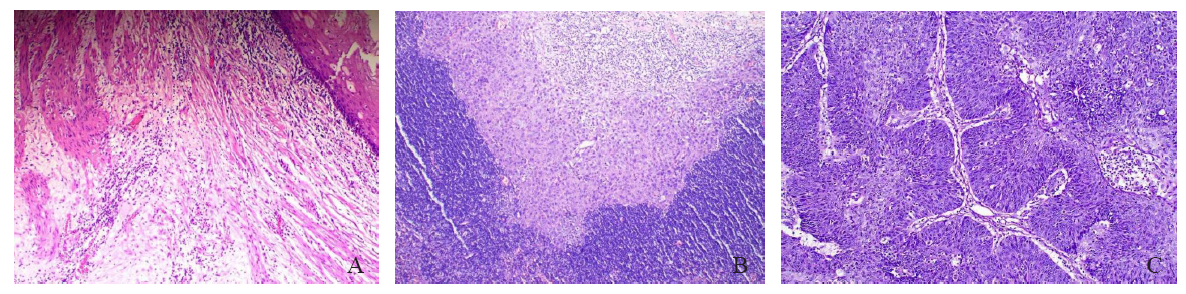
Fig. 1
Microscopic pathology picture of patients with different levels of pathologic remission A: Microscopically, no distribution of tumor cells was seen, and the esophagus was structurally normal, with a complex flat epithelium in the mucosa and connective tissue in the submucosal layer, slight edema, and a high distribution of inflammatory cells. No other obvious pathologic changes were seen (H-E staining, ×40). B: Microscopically, a small number of incompletely differentiated squamous epithelioid tumor cells were seen in an amorphous distribution, loosely arranged and disordered, with pale stained cytoplasm. The surrounding blood vessels and connective tissue were hyperplastic, accompanied by a large number of inflammatory cells infiltration. On the other side, a large number of lymphocytes were also seen, which were densely arranged (H-E staining, ×40). C: Microscopically, large squamous epithelioid tumor cells were seen to be distributed, forming multiple finger-like projections with fibrovascular axes, and the tumor cells were arranged in an orderly fenestrated structure similar to that of the normal esophageal mucosa, but with a loss of cellular polarity, with nuclear schizophrenia prevalent, and surrounded by a scattering inflammatory cell infiltrate (H-E staining, ×100)."
Tab. 2
Comparison of clinical and treatment-related data among patients of TRG1, TRG2 and TRG3-4 groups [n (%)]"
| Data | TRG1 (n=14) | TRG2 (n=11) | TRG3-4 (n=37) | P value |
|---|---|---|---|---|
| Gender | 1.000 | |||
| Male | 11 (78.57) | 9 (81.82) | 28 (75.68) | |
| Female | 3 (21.43) | 2 (18.18) | 9 (24.32) | |
| Age/year x±s | 64.36±7.45 | 65.18±10.04 | 63.81±7.97 | 0.886 |
| BMI/(kg·m-2) x±s | 22.83±2.82 | 21.68±3.26 | 21.58±3.31 | 0.457 |
| Location | 0.342 | |||
| Upper | 3 (21.43) | 0 (0.00) | 4 (10.81) | |
| Middle | 10 (71.43) | 9 (81.82) | 23 (62.16) | |
| Lower | 1 (7.14) | 2 (18.18) | 10 (27.03) | |
| cT | 0.255 | |||
| cT2 | 2 (14.29) | 1 (9.09) | 3 (8.11) | |
| cT3 | 4 (28.57) | 6 (54.55) | 23 (62.16) | |
| cT4 | 8 (57.14) | 4 (36.36) | 11 (29.13) | |
| cN | 0.457 | |||
| Negative | 6 (42.86) | 2 (18.18) | 13 (35.14) | |
| Positive | 8 (57.14) | 9 (81.82) | 24 (64.86) | |
| cTNM | 0.358 | |||
| Ⅱ | 2 (14.29) | 1 (9.09) | 4 (10.81) | |
| Ⅲ | 4 (28.57) | 7 (63.64) | 21 (56.76) | |
| ⅣA | 8 (57.14) | 3 (27.27) | 12 (32.43) | |
| Chemotherapy | 1.000 | |||
| Paclitaxel+platinum | 13 (92.86) | 11 (100.00) | 34 (91.89) | |
| 5-FU+platinum | 1 (7.14) | 0 (0.00) | 3 (8.11) | |
| PD-1 | 0.283 | |||
| Sindilizumab | 13 (92.86) | 7 (63.64) | 27 (72.97) | |
| Tirelizumab | 0 | 2 (18.18) | 7 (18.92) | |
| Karelizumab | 1 (7.14) | 2 (18.18) | 3 (8.11) | |
| Treatment order | 0.590 | |||
| Chemotherapy-first | 10 (71.43) | 10 (90.91) | 25 (67.57) | |
| Immunotherapy-first | 4 (28.57) | 1 (9.09) | 9 (24.32) | |
| At the same time | 0 (0.00) | 0 (0.00) | 3 (8.10) | |
| Interval time/week x±s | 5.74±1.56 | 4.91±0.96 | 5.45±1.49 | 0.353 |
Tab. 3
Comparison of selected blood, inflammation and nutritional indices before neoadjuvant therapy in patients of TRG1, TRG2 and TRG3-4 groups ($\bar{x}±s$)"
| Factor | TRG1 group (n=14) | TRG2 group (n=11) | TRG3-4 group (n=37) | P value |
|---|---|---|---|---|
| Hb/(g/L) | 126.86±11.88 | 117.36±14.15 | 126.57±15.78 | 0.175 |
| PLT/(×109/L) | 219.93±50.38 | 209.45±45.20 | 191.62±53.67 | 0.191 |
| neutrophil/(×109/L) | 4.76±1.80 | 4.91±1.72 | 4.53±2.28 | 0.848 |
| lymphocyte/(×109/L) | 1.57±0.43 | 1.77±0.42 | 1.46±0.39 | 0.081 |
| eosinophil/(×109/L) | 0.24±0.25 | 0.24±0.22 | 0.20±0.16 | 0.723 |
| albumin/(g/L) | 39.60±2.99 | 39.63±1.86 | 39.34±3.30 | 0.943 |
| PDW/% | 16.20±0.30 | 15.92±0.61 | 16.26±0.30 | 0.032 |
| RDW/% | 12.86±0.68 | 13.64±1.58 | 13.06±0.75 | 0.111 |
| NLR | 3.55±2.78 | 2.86±1.00 | 3.25±1.64 | 0.662 |
| PLR | 157.50±78.73 | 121.95±29.54 | 140.00±55.08 | 0.319 |
| LMR | 3.95±2.28 | 3.96±1.45 | 3.56±1.29 | 0.642 |
| SII | 807.63±763.90 | 607.36±264.59 | 639.19±399.35 | 0.490 |
| PNI | 47.43±4.01 | 48.49±2.71 | 46.63±4.03 | 0.356 |
| GNRI | 101.63±7.49 | 100.53±7.22 | 100.58±8.29 | 0.908 |
Tab. 4
Comparison of selected preoperative blood, inflammation and nutritional indices after neoadjuvant therapy before surgery in patients of TRG1, TRG2 and TRG3-4 groups ($\bar{x}±s$)"
| Factor | TRG1 group (n=14) | TRG2 group (n=11) | TRG3-4 group (n=37) | P value |
|---|---|---|---|---|
| Hb/(g/L) | 118.79±12.57 | 117.82±13.84 | 116.08±13.56 | 0.794 |
| PLT/(×109/L) | 208.71±67.93 | 216.73±70.94 | 184.65±71.39 | 0.314 |
| neutrophil/(×109/L) | 2.97±0.67 | 2.32±1.01 | 3.06±1.04 | 0.091 |
| lymphocyte/(×109/L) | 1.41±0.44 | 1.70±0.42 | 1.36±0.44 | 0.079 |
| eosinophil/(×109/L) | 0.25±0.21 | 0.11±0.07 | 0.19±0.27 | 0.343 |
| albumin/(g/L) | 39.51±2.53 | 40.94±2.50 | 39.81±3.18 | 0.444 |
| PDW/% | 16.11±0.50 | 16.02±0.55 | 16.22±0.37 | 0.344 |
| RDW/% | 15.84±1.64 | 16.88±3.36 | 15.46±1.85 | 0.163 |
| NLR | 2.21±0.55 | 1.43±0.80 | 2.56±1.47 | 0.031 |
| PLR | 160.53±71.53 | 131.68±45.77 | 151.62±80.27 | 0.613 |
| LMR | 3.54±1.05 | 4.02±1.54 | 3.73±2.10 | 0.811 |
| SII | 473.75±267.10 | 326.58±242.90 | 480.42±318.12 | 0.309 |
| PNI | 46.56±3.40 | 49.46±4.05 | 46.55±4.24 | 0.105 |
| GNRI | 101.49±7.73 | 100.53±7.22 | 100.58±8.29 | 0.920 |
Tab. 5
Intra-group comparison of PDW before neoadjuvant therapy in patients of TRG1, TRG2 and TRG3-4 groups"
| Comparison of different grades | PDW (%) before neoadjuvant therapy | P value |
|---|---|---|
| TRG1 vs TRG2 | (16.20±0.30) vs (15.92±0.61) | 0.064 |
| TRG1 vs TRG3-4 | (16.20±0.30) vs (16.26±0.30) | 0.595 |
| TRG2 vs TRG3-4 | (15.92±0.61) vs (16.26±0.30) | 0.009 |
Tab. 6
Intra-group comparison of NLR after neoadjuvant therapy before surgery in patients of TRG1, TRG2 and TRG3-4 groups"
| Comparison of different grades | NLR after neoadjuvant therapy before surgery | P value |
|---|---|---|
| TRG1 vs TRG2 | (2.21±0.55) vs (1.43±0.80) | 0.120 |
| TRG1 vs TRG3-4 | (2.21±0.55) vs (2.56±1.47) | 0.355 |
| TRG2 vs TRG3-4 | (1.43±0.80) vs (2.56±1.47) | 0.009 |
| [1] | SUNG H, FERLAY J, SIEGEL R L, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2021, 71(3): 209-249. |
| [2] |
SMYTH E C, LAGERGREN J, FITZGERALD R C, et al. Oesophageal cancer[J]. Nat Rev Dis Primers, 2017, 3: 17048.
doi: 10.1038/nrdp.2017.48 pmid: 28748917 |
| [3] | 刘宗超, 李哲轩, 张阳, 等. 2020全球癌症统计报告解读[J]. 肿瘤综合治疗电子杂志, 2021, 7(2): 1-14. |
| LIU Z C, LI Z X, ZHANG Y, et al. Interpretation on the report of global cancer statistics 2020[J]. J Multidiscip Cancer Manag Electron Version, 2021, 7(2): 1-14. | |
| [4] |
MALTHANER R, WONG R K S, SPITHOFF K, et al. Preoperative or postoperative therapy for resectable oesophageal cancer: an updated practice guideline[J]. Clin Oncol (R Coll Radiol), 2010, 22(4): 250-256.
doi: 10.1016/j.clon.2010.02.005 pmid: 20398848 |
| [5] |
RICE T W, RUSCH V W, APPERSON-HANSEN C, et al. Worldwide esophageal cancer collaboration[J]. Dis Esophagus, 2009, 22(1): 1-8.
doi: 10.1111/j.1442-2050.2008.00901.x pmid: 19196264 |
| [6] |
ANDO N, KATO H, IGAKI H, et al. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907)[J]. Ann Surg Oncol, 2012, 19(1): 68-74.
doi: 10.1245/s10434-011-2049-9 pmid: 21879261 |
| [7] |
SHAPIRO J, VAN LANSCHOT J J B, HULSHOF M C C M, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial[J]. Lancet Oncol, 2015, 16(9): 1090-1098.
doi: S1470-2045(15)00040-6 pmid: 26254683 |
| [8] |
JOYCE J A, FEARON D T. T cell exclusion, immune privilege, and the tumor microenvironment[J]. Science, 2015, 348(6230): 74-80.
doi: 10.1126/science.aaa6204 pmid: 25838376 |
| [9] | KOJIMA T, SHAH M A, MURO K, et al. Randomized phase Ⅲ KEYNOTE-181 study of pembrolizumab versus chemotherapy in advanced esophageal cancer[J]. J Clin Oncol, 2020, 38(35): 4138-4148. |
| [10] |
KATO K, CHO B C, TAKAHASHI M, et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, randomised, open-label, phase 3 trial[J]. Lancet Oncol, 2019, 20(11): 1506-1517.
doi: S1470-2045(19)30626-6 pmid: 31582355 |
| [11] | HUANG J, XU J M, CHEN Y, et al. Camrelizumab versus investigator’s choice of chemotherapy as second-line therapy for advanced or metastatic oesophageal squamous cell carcinoma (ESCORT): a multicentre, randomised, open-label, phase 3 study[J]. Lancet Oncol, 2020, 21(6): 832-842. |
| [12] | DE CASTRO JUNIOR G, SEGALLA J G, DE AZEVEDO S J, et al. A randomised phase Ⅱ study of chemoradiotherapy with or without nimotuzumab in locally advanced oesophageal cancer: NICE trial[J]. Eur J Cancer, 2018, 88: 21-30. |
| [13] |
ZHANG Z Y, HONG Z N, XIE S H, et al. Neoadjuvant sintilimab plus chemotherapy for locally advanced esophageal squamous cell carcinoma: a single-arm, single-center, phase 2 trial (ESONICT-1)[J]. Ann Transl Med, 2021, 9(21): 1623.
doi: 10.21037/atm-21-5381 pmid: 34926667 |
| [14] |
LI C Q, ZHAO S G, ZHENG Y Y, et al. Preoperative pembrolizumab combined with chemoradiotherapy for oesophageal squamous cell carcinoma (PALACE-1)[J]. Eur J Cancer, 2021, 144: 232-241.
doi: 10.1016/j.ejca.2020.11.039 pmid: 33373868 |
| [15] |
SHAH M A, KENNEDY E B, CATENACCI D V, et al. Treatment of locally advanced esophageal carcinoma: ASCO guideline[J]. J Clin Oncol, 2020, 38(23): 2677-2694.
doi: 10.1200/JCO.20.00866 pmid: 32568633 |
| [16] | LIN J W, HSU C P, YEH H L, et al. The impact of pathological complete response after neoadjuvant chemoradiotherapy in locally advanced squamous cell carcinoma of esophagus[J]. J Chin Med Assoc, 2018, 81(1): 18-24. |
| [17] |
CHEVROLLIER G S, GIUGLIANO D N, PALAZZO F, et al. Patients with non-response to neoadjuvant chemoradiation for esophageal cancer have no survival advantage over patients undergoing primary esophagectomy[J]. J Gastrointest Surg, 2020, 24(2): 288-298.
doi: 10.1007/s11605-019-04161-9 pmid: 30809782 |
| [18] | AL-KAABI A, VAN DER POST R S, VAN DER WERF L R, et al. Impact of pathological tumor response after CROSS neoadjuvant chemoradiotherapy followed by surgery on long-term outcome of esophageal cancer: a population-based study[J]. Acta Oncol, 2021, 60(4): 497-504. |
| [19] |
RICE T W, ISHWARAN H, FERGUSON M K, et al. Cancer of the esophagus and esophagogastric junction: an eighth edition staging primer[J]. J Thorac Oncol, 2017, 12(1): 36-42.
doi: S1556-0864(16)31235-7 pmid: 27810391 |
| [20] | 国家卫生健康委员会. 食管癌诊疗规范(2018年版)[J]. 中华消化病与影像杂志(电子版), 2019, 9(4): 158-192. |
| National Health Commission. Diagnostic and therapeutic criteria for esophageal cancer (2018 edition)[J]. Chin J Dig Med Imageology Electron Ed, 2019, 9(4): 158-192. | |
| [21] |
MANDARD A M, DALIBARD F, MANDARD J C, et al. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations[J]. Cancer, 1994, 73(11): 2680-2686.
doi: 10.1002/1097-0142(19940601)73:11<2680::aid-cncr2820731105>3.0.co;2-c pmid: 8194005 |
| [22] |
BASCH E. New frontiers in patient-reported outcomes: adverse event reporting, comparative effectiveness, and quality assessment[J]. Annu Rev Med, 2014, 65: 307-317.
doi: 10.1146/annurev-med-010713-141500 pmid: 24274179 |
| [23] | 姚鹏, 别俊, 李俊峰, 等. 信迪利单抗联合白蛋白紫杉醇+奈达铂化疗用于局部晚期食管癌术前新辅助治疗的临床观察[J]. 四川医学, 2023, 44(6): 579-584. |
| YAO P, BIE J, LI J F, et al. Clinical observation of sintilimab combined with albumin-bound paclitaxel and nedaplatin in preoperative neoadjuvant therapy for locally advanced esophageal cancer[J]. Sichuan Med J, 2023, 44(6): 579-584. | |
| [24] |
WU Z G, ZHENG Q, CHEN H Q, et al. Efficacy and safety of neoadjuvant chemotherapy and immunotherapy in locally resectable advanced esophageal squamous cell carcinoma[J]. J Thorac Dis, 2021, 13(6): 3518-3528.
doi: 10.21037/jtd-21-340 pmid: 34277047 |
| [25] | YANG W X, XING X B, YEUNG S J, et al. Neoadjuvant programmed cell death 1 blockade combined with chemotherapy for resectable esophageal squamous cell carcinoma[J]. J Immunother Cancer, 2022, 10(1): e003497. |
| [26] | LIU J, LI J P, LIN W L, et al. Neoadjuvant camrelizumab plus chemotherapy for resectable, locally advanced esophageal squamous cell carcinoma (NIC-ESCC2019): a multicenter, phase 2 study[J]. Int J Cancer, 2022, 151(1): 128-137. |
| [27] |
DENG J H, ZHANG P, SUN Y, et al. Prognostic and clinicopathological significance of platelet to lymphocyte ratio in esophageal cancer: a meta-analysis[J]. J Thorac Dis, 2018, 10(3): 1522-1531.
doi: 10.21037/jtd.2018.02.58 pmid: 29707302 |
| [28] |
SUN Y G, ZHANG L F. The clinical use of pretreatment NLR, PLR, and LMR in patients with esophageal squamous cell carcinoma: evidence from a meta-analysis[J]. Cancer Manag Res, 2018, 10: 6167-6179.
doi: 10.2147/CMAR.S171035 pmid: 30538564 |
| [29] |
ZHANG H D, SHANG X B, REN P, et al. The predictive value of a preoperative systemic immune-inflammation index and prognostic nutritional index in patients with esophageal squamous cell carcinoma[J]. J Cell Physiol, 2019, 234(2): 1794-1802.
doi: 10.1002/jcp.27052 pmid: 30070689 |
| [30] |
OKADOME K, BABA Y, YAGI T, et al. Prognostic nutritional index, tumor-infiltrating lymphocytes, and prognosis in patients with esophageal cancer[J]. Ann Surg, 2020, 271(4): 693-700.
doi: 10.1097/SLA.0000000000002985 pmid: 30308614 |
| [31] |
DONSKOV F. Immunomonitoring and prognostic relevance of neutrophils in clinical trials[J]. Semin Cancer Biol, 2013, 23(3): 200-207.
doi: 10.1016/j.semcancer.2013.02.001 pmid: 23403174 |
| [32] |
BALKWILL F, MANTOVANI A. Inflammation and cancer: back to Virchow?[J]. Lancet, 2001, 357(9255): 539-545.
doi: 10.1016/S0140-6736(00)04046-0 pmid: 11229684 |
| [33] | KIDANE D, CHAE W J, CZOCHOR J, et al. Interplay between DNA repair and inflammation, and the link to cancer[J]. Crit Rev Biochem Mol Biol, 2014, 49(2): 116-139. |
| [34] | MIERKE C T. The fundamental role of mechanical properties in the progression of cancer disease and inflammation[J]. Rep Prog Phys, 2014, 77(7): 076602. |
| [35] |
LABIANO S, PALAZON A, MELERO I. Immune response regulation in the tumor microenvironment by hypoxia[J]. Semin Oncol, 2015, 42(3): 378-386.
doi: 10.1053/j.seminoncol.2015.02.009 pmid: 25965356 |
| [36] |
MEI Z B, SHI L, WANG B, et al. Prognostic role of pretreatment blood neutrophil-to-lymphocyte ratio in advanced cancer survivors: a systematic review and meta-analysis of 66 cohort studies[J]. Cancer Treat Rev, 2017, 58: 1-13.
doi: S0305-7372(17)30085-3 pmid: 28602879 |
| [37] | TEMPLETON A J, MCNAMARA M G, ŠERUGA B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis[J]. J Natl Cancer Inst, 2014, 106(6): dju124. |
| [38] | AKCE M, LIU Y, ZAKKA K, et al. Impact of sarcopenia, BMI, and inflammatory biomarkers on survival in advanced hepatocellular carcinoma treated with anti-PD-1 antibody[J]. Am J Clin Oncol, 2021, 44(2): 74-81. |
| [39] |
SEKINE K, KANDA S, GOTO Y, et al. Change in the lymphocyte-to-monocyte ratio is an early surrogate marker of the efficacy of nivolumab monotherapy in advanced non-small-cell lung cancer[J]. Lung Cancer, 2018, 124: 179-188.
doi: S0169-5002(18)30529-4 pmid: 30268458 |
| [40] |
XU J M, LI Y, FAN Q X, et al. Clinical and biomarker analyses of sintilimab versus chemotherapy as second-line therapy for advanced or metastatic esophageal squamous cell carcinoma: a randomized, open-label phase 2 study (ORIENT-2)[J]. Nat Commun, 2022, 13(1): 857.
doi: 10.1038/s41467-022-28408-3 pmid: 35165274 |
| [41] |
WU X B, HAN R K, ZHONG Y P, et al. Post treatment NLR is a predictor of response to immune checkpoint inhibitor therapy in patients with esophageal squamous cell carcinoma[J]. Cancer Cell Int, 2021, 21(1): 356.
doi: 10.1186/s12935-021-02072-x pmid: 34233686 |
| [42] | CHUA W, CHARLES K A, BARACOS V E, et al. Neutrophil/lymphocyte ratio predicts chemotherapy outcomes in patients with advanced colorectal cancer[J]. Br J Cancer, 2011, 104(8): 1288-1295. |
| [43] |
LI N, DIAO Z Y, HUANG X Y, et al. Increased platelet distribution width predicts poor prognosis in melanoma patients[J]. Sci Rep, 2017, 7(1): 2970.
doi: 10.1038/s41598-017-03212-y pmid: 28592835 |
| [44] |
ZHANG H, LIU L, FU S, et al. Higher platelet distribution width predicts poor prognosis in laryngeal cancer[J]. Oncotarget, 2017, 8(29): 48138-48144.
doi: 10.18632/oncotarget.18306 pmid: 28624815 |
| [45] |
CHENG S Q, HAN F Y, WANG Y, et al. The red distribution width and the platelet distribution width as prognostic predictors in gastric cancer[J]. BMC Gastroenterol, 2017, 17(1): 163.
doi: 10.1186/s12876-017-0685-7 pmid: 29262773 |
| [46] |
SONG Q, WU J Z, WANG S, et al. Elevated preoperative platelet distribution width predicts poor prognosis in esophageal squamous cell carcinoma[J]. Sci Rep, 2019, 9(1): 15234.
doi: 10.1038/s41598-019-51675-y pmid: 31645619 |
| [47] |
DUAN H T, WANG T H, LUO Z L, et al. A multicenter single-arm trial of sintilimab in combination with chemotherapy for neoadjuvant treatment of resectable esophageal cancer (SIN-ICE study)[J]. Ann Transl Med, 2021, 9(22): 1700.
doi: 10.21037/atm-21-6102 pmid: 34988209 |
| [48] | DUAN H T, SHAO C J, PAN M H, et al. Neoadjuvant pembrolizumab and chemotherapy in resectable esophageal cancer: an open-label, single-arm study (PEN-ICE)[J]. Front Immunol, 2022, 13: 849984. |
| [49] | YAN X L, DUAN H T, NI Y F, et al. Tislelizumab combined with chemotherapy as neoadjuvant therapy for surgically resectable esophageal cancer: a prospective, single-arm, phase Ⅱ study (TD-NICE)[J]. Int J Surg, 2022, 103: 106680. |
| [50] | SALAS-BENITO D, PÉREZ-GRACIA J L, PONZ-SARVISÉ M, et al. Paradigms on immunotherapy combinations with chemotherapy[J]. Cancer Discov, 2021, 11(6): 1353-1367. |
| [51] | TOPALIAN S L, TAUBE J M, PARDOLL D M. Neoadjuvant checkpoint blockade for cancer immunotherapy[J]. Science, 2020, 367(6477): eaax0182. |
| [52] | YAO W, ZHAO X, GONG Y, et al. Impact of the combined timing of PD-1/PD-L1 inhibitors and chemotherapy on the outcomes in patients with refractory lung cancer[J]. ESMO Open, 2021, 6(2): 100094. |
| [53] | XING W Q, ZHAO L D, ZHENG Y, et al. The sequence of chemotherapy and toripalimab might influence the efficacy of neoadjuvant chemoimmunotherapy in locally advanced esophageal squamous cell cancer-a phase Ⅱ study[J]. Front Immunol, 2021, 12: 772450. |
| [54] | YANG Y, LIU J, LIU Z C, et al. Two-year outcomes of clinical N2-3 esophageal squamous cell carcinoma after neoadjuvant chemotherapy and immunotherapy from the phase 2 NICE study[J]. J Thorac Cardiovasc Surg, 2024, 167(3): 838-847.e1. |
| [55] | SHANG X B, ZHAO G, LIANG F, et al. Safety and effectiveness of pembrolizumab combined with paclitaxel and cisplatin as neoadjuvant therapy followed by surgery for locally advanced resectable (stage Ⅲ) esophageal squamous cell carcinoma: a study protocol for a prospective, single-arm, single-center, open-label, phase-Ⅱ trial (Keystone-001)[J]. Ann Transl Med, 2022, 10(4): 229. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
沪ICP备12009617
Powered by Beijing Magtech Co. Ltd
