Welcome to China Oncology,
China Oncology ›› 2024, Vol. 34 ›› Issue (12): 1067-1079.doi: 10.19401/j.cnki.1007-3639.2024.12.001
• Article • Previous Articles Next Articles
ZHANG Jian1,2, HAN Qian3, XU Fei4, GAN Lu5, CHEN Zhanhong6, MA Li7, WANG Hao8, LIU Jieqiong9, WU Xiaohong10, CAI Li11, ZHAO Bing12, LÜ Zheng13, LI Li14, NI Sujie15, HU Xichun1,2( )
)
Received:2024-11-13
Online:2024-12-30
Published:2025-01-21
Contact:
HU Xichun
Share article
ZHANG Jian, HAN Qian, XU Fei, GAN Lu, CHEN Zhanhong, MA Li, WANG Hao, LIU Jieqiong, WU Xiaohong, CAI Li, ZHAO Bing, LÜ Zheng, LI Li, NI Sujie, HU Xichun. Comprehensive management strategy of interstitial lung disease induced by trastuzumab deruxtecan[J]. China Oncology, 2024, 34(12): 1067-1079.
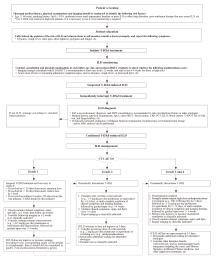
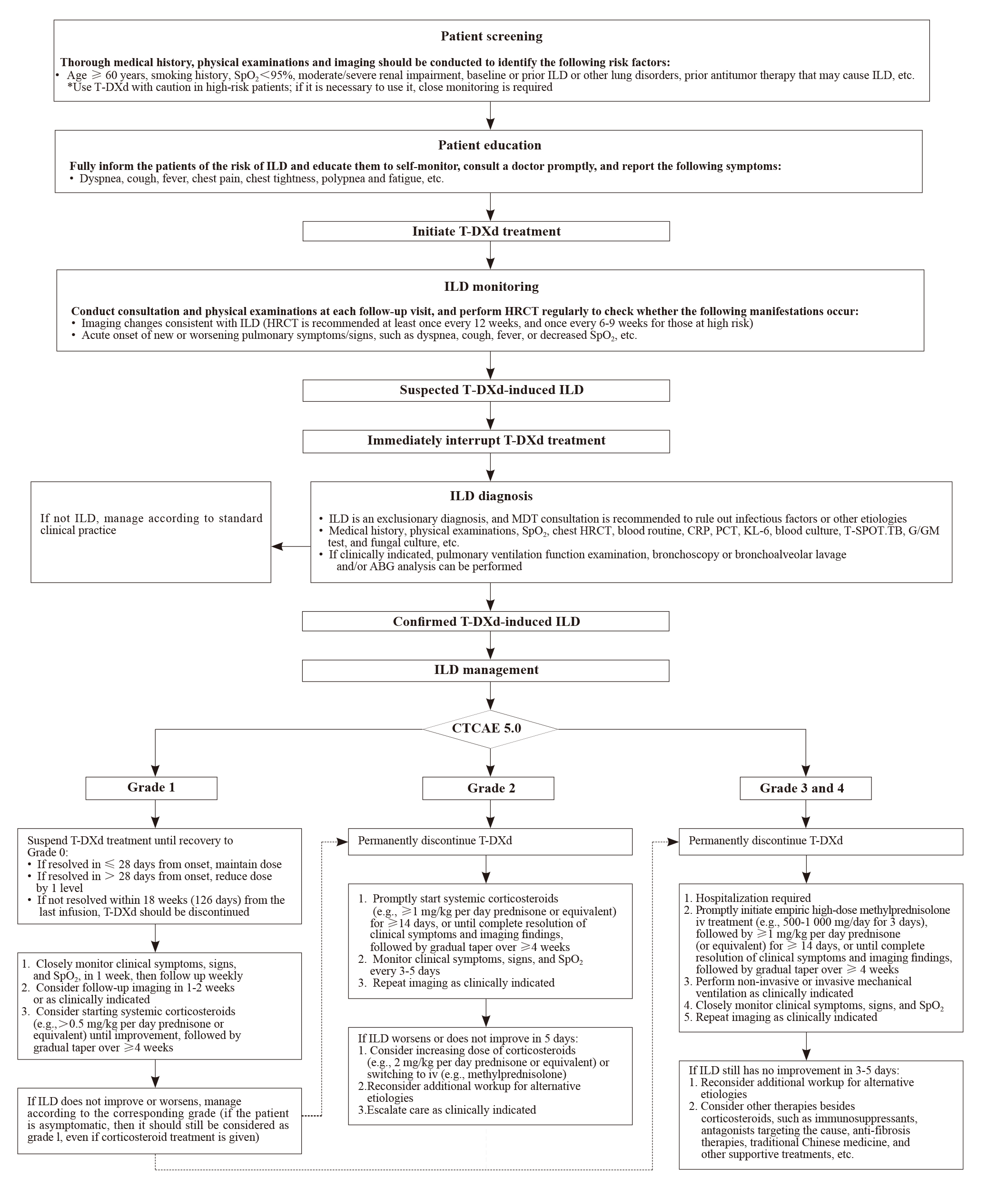
Fig. 1
Clinical pathway for comprehensive management of T-DXd-induced ILD ABG: Arterial blood gas; CRP: C-reactive protein; CTCAE: Common terminology criteria for adverse events; G: 1,3-β-D-glucan; GM: Galactomannan; HRCT: High-resolution computed tomography; ILD: Interstitial lung disease; iv: Intravenous injection; KL-6: Krebs von den Lungen-6; MDT: Multidisciplinary team; PCT: Procalcitonin; SpO2: Pulse oxygen saturation; T-DXd: Trastuzumab deruxtecan; T-SPOT.TB: T-cell assay for tuberculosis infection."
Tab. 1
CTCAE 5.0 grading criteria [40, 48]"
| Grading | Definition |
|---|---|
| Grade 1 | Asymptomatic; clinical or diagnostic observations only; intervention not indicated |
| Grade 2 | Symptomatic; medical intervention indicated; limiting instrumental ADL |
| Grade 3 | Severe symptoms; limiting self-care ADL; oxygen indicated (at rest, SpO2 < 88% or PaO2 ≤ 55 mmHg) |
| Grade 4 | Life-threatening respiratory compromise; urgent intervention indicated (e.g., tracheotomy or intubation) |
| Grade 5 | Death |
Tab. 2
ILD management standards in the T-DXd prescribing information and clinical study protocols [21,50-51]"
| Severity | Management measures |
|---|---|
| Asymptomatic ILD (grade 1) | Once ILD is suspected, corticosteroid treatment (e.g., ≥0.5 mg/kg per day prednisone or equivalent) should be considered immediatelyInterrupt T-DXd until ILD is resolved to grade 0, then:• If resolved in ≤28 days from onset, maintain dose• If resolved in >28 days from onset, reduce dose by 1 level (see |
| Symptomatic ILD (grade ≥2) | Permanently discontinue T-DXdOnce ILD is suspected, promptly initiate corticosteroids (e.g., ≥1 mg/kg per day prednisone or equivalent) for ≥14 days followed by a gradual taper over ≥4 weeks |
Tab. 3
T-DXd dosage adjustment schedule [50]"
| Dose reduction schedule | Breast cancer, NSCLC, and IHC 3+ solid tumors | Gastric cancer |
|---|---|---|
| Recommended starting dose | 5.4 mg/kg | 6.4 mg/kg |
| First dose reduction | 4.4 mg/kg | 5.4 mg/kg |
| Second dose reduction | 3.2 mg/kg | 4.4 mg/kg |
| Further dose reduction required | Discontinue treatment | Discontinue treatment |
| [1] | HURVITZ S A, HEGG R, CHUNG W P, et al. Trastuzumab deruxtecan versus trastuzumab emtansine in patients with HER2-positive metastatic breast cancer: updated results from DESTINY-Breast03, a randomised, open-label, phase 3 trial[J]. Lancet, 2023, 401(10371): 105-117. |
| [2] | CORTéS J, HURVITZ S A, IM S A, et al. Trastuzumab deruxtecan versus trastuzumab emtansine in HER2-positive metastatic breast cancer: long-term survival analysis of the DESTINY-Breast03 trial[J]. Nat Med, 2024, 30(8): 2208-2215. |
| [3] | MODI S, JACOT W, YAMASHITA T, et al. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer[J]. N Engl J Med, 2022, 387(1): 9-20. |
| [4] | SHEN L, CHEN P, LU J, et al. Trastuzumab deruxtecan (T-DXd) in Chinese patients (pts) with previously treated HER2-positive locally advanced/metastatic gastric cancer (GC) or gastroesophageal junction adenocarcinoma (GEJA): primary efficacy and safety from the phase Ⅱ single-arm DESTINY-Gastric06 (DG06) trial[J]. Ann Oncol, 2023, 34(supplement 4): S1542-S1543. |
| [5] | GOTO K, GOTO Y, KUBO T, et al. Trastuzumab deruxtecan in patients with HER2-mutant metastatic non-small cell lung cancer: primary results from the randomized, phase Ⅱ DESTINY-lung02 trial[J]. J Clin Oncol, 2023, 41(31): 4852-4863. |
| [6] | CHENG Y, WU L, FANG Y, et al. Trastuzumab deruxtecan (T-DXd) in Chinese patients (pts) with previously treated HER2 mutant non-small cell lung cancer (NSCLC): primary analysis from the phase 2 DESTINY-lung05 (DL-05) trial[J]. Cancer Res, 2024, 84(7_Supplement): CT248-CT248. |
| [7] | SPAGNOLO P, BONNIAUD P, ROSSI G, et al. Drug-induced interstitial lung disease[J]. Eur Respir J, 2022, 60(4): 2102776. |
| [8] | HENNING J W, BREZDEN-MASLEY C, GELMON K, et al. Managing the risk of lung toxicity with trastuzumab deruxtecan (T-DXd): a Canadian perspective[J]. Curr Oncol, 2023, 30(9): 8019-8038. |
| [9] | 抗肿瘤药物相关间质性肺病诊治专家共识专家委员会. 抗肿瘤药物相关间质性肺病诊治专家共识[J]. 中华肿瘤杂志 2022, 44(7): 693-702. |
| Anticancer Drug-induced Interstitial Lung Disease Management Group. Expert consensus on the diagnosis and treatment of anticancer drug-induced interstitial lung disease[J]. Chin J Oncol, 2022, 44(7): 693-702. | |
| [10] | SAURA C, MODI S, KROP I, et al. Trastuzumab deruxtecan in previously treated patients with HER2-positive metastatic breast cancer: updated survival results from a phase Ⅱ trial (DESTINY-Breast01)[J]. Ann Oncol, 2024, 35(3): 302-307. |
| [11] | KIM S B, ANDRÉ F, TAKANO T, et al. Trastuzumab deruxtecan (T-DXd) vs treatment of physician's choice (TPC) in patients (pts) with HER2+ metastatic breast cancer (mBC) previously treated with trastuzumab emtansine (T-DM1): updated overall survival (OS) results of the randomized phase Ⅲ DESTINY-breast (DB-)02 study[J]. ESMO Open, 2024, 9(supplement 4): 103204. |
| [12] | MODI S, JACOT W, IWATA H, et al. Trastuzumab deruxtecan (T-DXd) versus treatment of physician's choice (TPC) in patients (pts) with HER2- low unresectable and/or metastatic breast cancer (mBC): updated survival results of the randomized, phase Ⅲ DESTINY-Breast04 study[J]. Ann Oncol, 2023, 34: S334-S335. |
| [13] | PARK Y H, JACOT W, HURVITZ S A, et al. Exploratory pooled safety analysis of trastuzumab deruxtecan (T-DXd) in patients with HER2+ or HER2- low unresectable and/or metastatic breast cancer (mBC) in DESTINY-Breast trials[J]. ESMO Open, 2024, 9(supplement 4): 103208. |
| [14] | BARDIA A, HU X, DENT R, et al. Trastuzumab deruxtecan after endocrine therapy in metastatic breast cancer[J]. N Engl J Med, 2024. Online ahead of print. |
| [15] | HARBECK N, CIRUELOS E, JERUSALEM G, et al. Trastuzumab deruxtecan in HER2-positive advanced breast cancer with or without brain metastases: a phase 3b/4 trial[J]. Nat Med, 2024. Online ahead of print. |
| [16] |
LI B T, MERIC-BERNSTAM F, BARDIA A, et al. Trastuzumab deruxtecan in patients with solid tumours harbouring specific activating HER2 mutations (DESTINY-PanTumor01): an international, phase 2 study[J]. Lancet Oncol, 2024, 25(6): 707-719.
doi: 10.1016/S1470-2045(24)00140-2 pmid: 38710187 |
| [17] | MERIC-BERNSTAM F, MAKKER V, OAKNIN A, et al. Efficacy and safety of trastuzumab deruxtecan in patients with HER2-expressing solid tumors: primary results from the DESTINY-PanTumor02 phase Ⅱ trial[J]. J Clin Oncol, 2024, 42(1): 47-58. |
| [18] | SHITARA K, BANG Y J, IWASA S, et al. Trastuzumab deruxtecan in previously treated HER2-positive gastric cancer[J]. N Engl J Med, 2020, 382(25): 2419-2430. |
| [19] |
VAN CUTSEM E, DI BARTOLOMEO M, SMYTH E, et al. Trastuzumab deruxtecan in patients in the USA and Europe with HER2-positive advanced gastric or gastroesophageal junction cancer with disease progression on or after a trastuzumab-containing regimen (DESTINY-Gastric02): primary and updated analyses from a single-arm, phase 2 study[J]. Lancet Oncol, 2023, 24(7): 744-756.
doi: 10.1016/S1470-2045(23)00215-2 pmid: 37329891 |
| [20] |
RAGHAV K, SIENA S, TAKASHIMA A, et al. Trastuzumab deruxtecan in patients with HER2-positive advanced colorectal cancer (DESTINY-CRC02): primary results from a multicentre, randomised, phase 2 trial[J]. Lancet Oncol, 2024, 25(9): 1147-1162.
doi: 10.1016/S1470-2045(24)00380-2 pmid: 39116902 |
| [21] | CORTÉS J, KIM S B, CHUNG W P, et al. Trastuzumab deruxtecan versus trastuzumab emtansine for breast cancer[J]. N Engl J Med, 2022, 386(12): 1143-1154. |
| [22] | TARANTINO P, TOLANEY S M. Detecting and managing T-DXd-related interstitial lung disease: the five "S" rules[J]. JCO Oncol Pract, 2023, 19(8): 526-527. |
| [23] | ALTHOBIANI M A, RUSSELL A M, JACOB J, et al. Interstitial lung disease: a review of classification, etiology, epidemiology, clinical diagnosis, pharmacological and non-pharmacological treatment[J]. Front Med (Lausanne), 2024, 11: 1296890. |
| [24] | SKEOCH S, WEATHERLEY N, SWIFT A J, et al. Drug-induced interstitial lung disease: a systematic review[J]. J Clin Med, 2018, 7(10): 356. |
| [25] |
KAKU S, HORINOUCHI H, WATANABE H, et al. Incidence and prognostic factors in severe drug-induced interstitial lung disease caused by antineoplastic drug therapy in the real world[J]. J Cancer Res Clin Oncol, 2022, 148(7): 1737-1746.
doi: 10.1007/s00432-022-03932-3 pmid: 35129672 |
| [26] | ZHU Z, SHEN G, LI J, et al. Incidence of antibody-drug conjugates-related pneumonitis in patients with solid tumors: a systematic review and meta-analysis[J]. Crit Rev Oncol Hematol, 2023, 184: 103960. |
| [27] | CONTE P, ASCIERTO P A, PATELLI G, et al. Drug-induced interstitial lung disease during cancer therapies: expert opinion on diagnosis and treatment[J]. ESMO Open, 2022, 7(2): 100404. |
| [28] | MATSUNO O. Drug-induced interstitial lung disease: mechanisms and best diagnostic approaches[J]. Respir Res, 2012, 13(1): 39. |
| [29] | MAHALINGAIAH P K, CIURLIONIS R, DURBIN K R, et al. Potential mechanisms of target-independent uptake and toxicity of antibody-drug conjugates[J]. Pharmacol Ther, 2019, 200: 110-125. |
| [30] |
TARANTINO P, MODI S, TOLANEY S M, et al. Interstitial lung disease induced by anti-ERBB2 antibody-drug conjugates: a review[J]. JAMA Oncol, 2021, 7(12): 1873-1881.
doi: 10.1001/jamaoncol.2021.3595 pmid: 34647966 |
| [31] | 德曲妥珠单抗临床管理路径及不良反应处理共识专家组. 德曲妥珠单抗临床管理路径及不良反应处理中国专家共识(2024版)[J]. 中华肿瘤杂志, 2024, (04): 304-318. |
| Trastuzumab Deruxtecan Clinical Management Pathwayand Adverse Reaction Management Consensus Expert Group. Chinese expert consensus on the management of clinical pathway and adverse events of trastuzumab deruxtecan (2024 edition)[J]. Chin J Oncol, 2024, (04): 304-318. | |
| [32] | ELU L Trastuzumab deruxctecan (T-DXd) associated interstitial lung disease (ILD) in a large real-world French cohort of patients with HER2-driven breast cancer and other malignancies[J]. Ann Oncol, 2024, 35(suppl_2): S357-S405. |
| [33] |
SCHWAIBLMAIR M, BEHR W, HAECKEL T, et al. Drug induced interstitial lung disease[J]. Open Respir Med J, 2012, 6: 63-74.
doi: 10.2174/1874306401206010063 pmid: 22896776 |
| [34] | SWAIN S M, NISHINO M, LANCASTER L H, et al. Multidisciplinary clinical guidance on trastuzumab deruxtecan (T-DXd)-related interstitial lung disease/pneumonitis-focus on proactive monitoring, diagnosis, and management[J]. Cancer Treat Rev, 2022, 106: 102378. |
| [35] |
YIN O, IWATA H, LIN C C, et al. Exposure-response relationships in patients with HER2-positive metastatic breast cancer and other solid tumors treated with trastuzumab deruxtecan[J]. Clin Pharmacol Ther, 2021, 110(4): 986-996.
doi: 10.1002/cpt.2291 pmid: 33999422 |
| [36] | WEKKING D, PORCU M, PELLEGRINO B, et al. Multidisciplinary clinical guidelines in proactive monitoring, early diagnosis, and effective management of trastuzumab deruxtecan (T-DXd)-induced interstitial lung disease (ILD) in breast cancer patients[J]. ESMO Open, 2023, 8(6): 102043. |
| [37] | CHIU J W Y, LEE S C, HO J C, et al. Clinical guidance on the monitoring and management of trastuzumab deruxtecan (T-DXd)-related adverse events: insights from an Asia-Pacific multidisciplinary panel[J]. Drug Saf, 2023, 46(10): 927-949. |
| [38] |
BARDIA A, HARNDEN K, MAURO L, et al. Clinical practices and institutional protocols on prophylaxis, monitoring, and management of selected adverse events associated with trastuzumab deruxtecan[J]. Oncologist, 2022, 27(8): 637-645.
doi: 10.1093/oncolo/oyac107 pmid: 35642907 |
| [39] | MOORE H, SHOFER S, GUISINGER A, et al. Abstract PS13-33: Feasibility of a comprehensive monitoring protocol for the prevention and treatment of interstitial lung disease in patients undergoing treatment with fam-trastuzumab deruxtecan[J]. Cancer Res, 2021, 81(4_Suppl): PS13-33. |
| [40] | 张剑, 沈维娜, 季冬梅, 等. 实体瘤中靶向药所致间质性肺病管理的复旦大学附属肿瘤医院标准[J]. 中国癌症杂志, 2021, 31(4): 241-249. |
|
ZHANG J, SHEN WN, JI DM, et al. FUSCC criteria for the management of targeted drug-induced interstitial lung disease in solid tumors[J]. Chin Oncol, 2021, 31(4): 241-249.
doi: 10.19401/j.cnki.1007-3639.2021.04.001 |
|
| [41] | RUGO H S, CROSSNO C L, GESTHALTER Y B, et al. Real-world perspectives and practices for pneumonitis/interstitial lung disease associated with trastuzumab deruxtecan use in human epidermal growth factor receptor 2-expressing metastatic breast cancer[J]. JCO Oncol Pract, 2023, 19(8): 539-546. |
| [42] | 中国医师协会肿瘤医师分会乳腺癌学组, 中国抗癌协会国际医疗交流分会. 中国乳腺癌抗体药物偶联物安全性管理专家共识[J]. 中华肿瘤杂志, 2022, 44(9): 913-927. |
| Breast Cancer Group, Branch of Oncologist, Chinese Medical Doctor Association. Chinese expert consensus of antibody-drug conjugate toxicity management for breast cancer[J]. Chin J Oncol, 2022, 44(9): 913-927. | |
| [43] |
CIRUELOS E, GARCÍA-SÁENZ J, GAVILá J, et al. Safety profile of trastuzumab deruxtecan in advanced breast cancer: expert opinion on adverse event management[J]. Clin Transl Oncol, 2024, 26(7): 1539-1548.
doi: 10.1007/s12094-024-03383-x pmid: 38336982 |
| [44] |
BABA T, KUSUMOTO M, KATO T, et al. Clinical and imaging features of interstitial lung disease in cancer patients treated with trastuzumab deruxtecan[J]. Int J Clin Oncol, 2023, 28(12): 1585-1596.
doi: 10.1007/s10147-023-02414-x pmid: 37787866 |
| [45] |
ZHENG P, LIU X, HUANG H, et al. Diagnostic value of KL-6 in idiopathic interstitial pneumonia[J]. J Thorac Dis, 2018, 10(8): 4724-4732.
doi: 10.21037/jtd.2018.07.54 pmid: 30233844 |
| [46] |
OHNISHI H, YOKOYAMA A, YASUHARA Y, et al. Circulating KL-6 levels in patients with drug induced pneumonitis[J]. Thorax, 2003, 58(10): 872-875.
doi: 10.1136/thorax.58.10.872 pmid: 14514942 |
| [47] | ZHANG T, SHEN P, DUAN C, et al. KL-6 as an Immunological biomarker predicts the severity, progression, acute exacerbation, and poor outcomes of interstitial lung disease: a systematic review and meta-analysis[J]. Front Immunol, 2021, 12: 745233. |
| [48] | NATIONAL CANCER INSTITUTE. Common terminology criteria for adverse events (CTCAE) v5.0[EB/OL]. 2017. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf. |
| [49] | RUGO H S, BIANCHINI G, CORTES J, et al. Optimizing treatment management of trastuzumab deruxtecan in clinical practice of breast cancer[J]. ESMO Open, 2022, 7(4): 100553. |
| [50] | Food and Drug Administration(fam-trastuzumab deruxtecan-nxki). prescribing information[EB/OL]. 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/761139s024lbl.pdf. |
| [51] | RUGO H S, JACOT W, TOKUNAGA E, et al. Trastuzumab deruxtecan (T-DXd) vs treatment of physician’s choice (TPC) in patients (pts) with HER2-low unresectable and/or metastatic breast cancer (mBC): a detailed safety analysis of the randomized, phase 3 DESTINY-Breast04 trial[J]. Ann Oncol, 2023, 8(1suppl_4): 101223-101223. |
| [52] | 韩茜, 罗群. 尼达尼布治疗纤维化性间质性肺疾病的研究综述[J]. 临床肺科杂志, 2023, 28(12): 1908-1914. |
| HAN Q, LUO Q. A review of trials of nintedanib in fibrotic interstitial lung disease[J]. J Clin Pulm Med, 2023, 28(12): 1908-1914. | |
| [53] | RUGO H S, TOKUNAGA E, IWATA H, et al. Pooled analysis of trastuzumab deruxtecan (T-DXd) retreatment (RTx) after recovery from grade (Gr) 1 interstitial lung disease/pneumonitis (ILD)[C]. ESMO Open, 9(suppl 4): 103326. |
| [54] | NATSUHARA K H, VELLA M, BEHR S C, et al. Treatment rechallenge after grade 1 trastuzumab-deruxtecan related interstitial lung disease: a real-world experience[J]. Ann Oncol 2024, 35(suppl_2): S357-S405. |
| [55] |
DE WEGER V A, SCHUTTE T, KONINGS I, et al. Successful trastuzumab-deruxtecan rechallenge after interstitial lung disease: a case report[J]. J Breast Cancer, 2023, 26(5): 519-523.
doi: 10.4048/jbc.2023.26.e38 pmid: 37926069 |
| [56] | NAM S, LIM S M, CHO B C, et al. Successful rechallenge of trastuzumab deruxtecan after drug-induced interstitial lung disease in a NSCLC with HER2 mutation: a case report[J]. JTO Clin Res Rep, 2024, 5(2): 100628. |
| [1] | Cancer Rehabilitation and Palliative Professional Committee of Shanghai Anti-Cancer Association , Cancer Drug Clinical Research Committee of Shanghai Anti-Cancer Association , Cancer Prevention and Clinical Research Committee of Chinese Aging Well Association . Shanghai expert consensus on whole-process management of antineoplastic-induced nausea and vomiting (2024 edition) [J]. China Oncology, 2024, 34(1): 104-134. |
| [2] | Energizing Specialty Construction-Multidisciplinary Expert Committee for Creating Healthy China, The Society of Breast Cancer China Anti-Cancer Association. Standardization of multi disciplinary team treatment for breast cancer (2023 edition) [J]. China Oncology, 2023, 33(12): 1188-1203. |
| [3] | Expert Committee of Nuclear Medicine, Chinese society of Clinical Oncology, Expert Committee of Thyroid Cancer, Chinese society of Clinical Oncology, Committee of Thyroid Disease Society, China International Exchange and Promotive Association for Medical and Health Care, Committee of Thyroid Disease Prevention and Treatment Society, China Population Culture Promotion Association. Expert consensus on management of differentiated thyroid carcinoma in children and adolescents (2022 edition) [J]. China Oncology, 2022, 32(5): 451-468. |
| [4] | Chinese Prostate Cancer Consortium (CPCC), YE Dingwei, HUANG Jian. Chinese Prostate Cancer Consortium (CPCC) Chinese expert consensus on advanced prostate cancer: clinical management of patients with metastatic hormone-sensitive prostate cancer treated by initial novel hormone therapy (2022 edition) [J]. China Oncology, 2022, 32(12): 1242-1258. |
| [5] | Onco-endocrinology Society of Chinese Anti-Cancer Association, Onco-endocrinology Committee of Chongqing Association of Integrative Medicine. Guidelines for the management of tumor-associated hyperglycemia (2021 edition) [J]. China Oncology, 2021, 31(7): 651-688. |
| [6] | ZHANG Jian , SHEN Weina , JI Dongmei , WANG Leiping , GONG Chengcheng , HU Xichun . FUSCC criteria for the management of targeted drug-induced interstitial lung disease in solid tumors [J]. China Oncology, 2021, 31(4): 241-249. |
| [7] | WU Qinan , TONG Nanwei . Interpretation of Guidelines for the Management of Tumor-associated Hyperglycemia (2021 edition) [J]. China Oncology, 2021, 31(12): 1153-1161. |
| [8] | YANG Ke, ZHENG Rong, LIN Yansong. The interpretation of management guidelines for children with thyroid nodules and differentiated thyroid cancer: radioactive iodine therapy and new progress [J]. China Oncology, 2019, 29(6): 401-411. |
| [9] | WU Xiaohua, ZHANG Jian, YIN Rutie, LOU Ge, GAO Yunong. Chinese expert experience in optimizing stage Ⅰ clinical study management of ovarian cancer [J]. China Oncology, 2019, 29(5): 321-327. |
| [10] | GU Xiaoli, CHENG Wenwu, CHEN Menglei, et al. The characteristics of Traditional Chinese Medicine syndrome and its relationship with survival time among patients with advanced cancer [J]. China Oncology, 2018, 28(7): 532-537. |
| [11] | LOU Feifei, XU Pingbo, HUANG Naisi, et al. Intraoperative anesthetic management in breast cancer patients undergoing free flap breast reconstruction [J]. China Oncology, 2016, 26(5): 383-387. |
| [12] | HU Qunchao, YU Xiaoli, GUO Xiaomao. Progress in the clinical use of radiotherapy for bone metastasis in breast cancer [J]. China Oncology, 2016, 26(4): 346-350. |
| [13] | NIE Xiaomeng, BAI Chong. The whole-process management of advanced non-small cell lung cancer according to the EGFR gene mutation state [J]. China Oncology, 2015, 25(5): 397-400. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
沪ICP备12009617
Powered by Beijing Magtech Co. Ltd
