Welcome to China Oncology,
China Oncology ›› 2025, Vol. 35 ›› Issue (11): 1067-1075.doi: 10.19401/j.cnki.1007-3639.2025.11.009
• Review • Previous Articles Next Articles
WANG Zhiling( ), CHEN Wanjin, CHENG Shengtao(
), CHEN Wanjin, CHENG Shengtao( )(
)( )
)
Received:2025-04-14
Revised:2025-07-15
Online:2025-11-30
Published:2025-12-12
Contact:
CHENG Shengtao
E-mail:shengtao@cqmu.edu.cn
Supported by:Share article
CLC Number:
WANG Zhiling, CHEN Wanjin, CHENG Shengtao. Research progress on the regulation of tumor malignancy by lactate[J]. China Oncology, 2025, 35(11): 1067-1075.
Fig. 1
Schematic diagram of lactate reshaping the metabolic process of tumor cells A: High concentration of lactate regulates metabolism; B: Lactate regulates energy metabolism; C: lactate regulates amino acid metabolism; D: Lactate regulates lipid metabolism. TCA: Tricarboxylic acid cycle; Glut1: Glucose transporter 1; PDH: Pyruvate dehydrogenase; MPRL13: Mitochondrial ribosomal protein L13; CLDN1: Claudin-1; LDH1: Lactate dehydrogenase 1; PCK2: Phosphoenolpyruvate carboxykinase 2; ASCT2: alanine-serine-cysteine transporter 2; GLS1: Glutaminase 1."
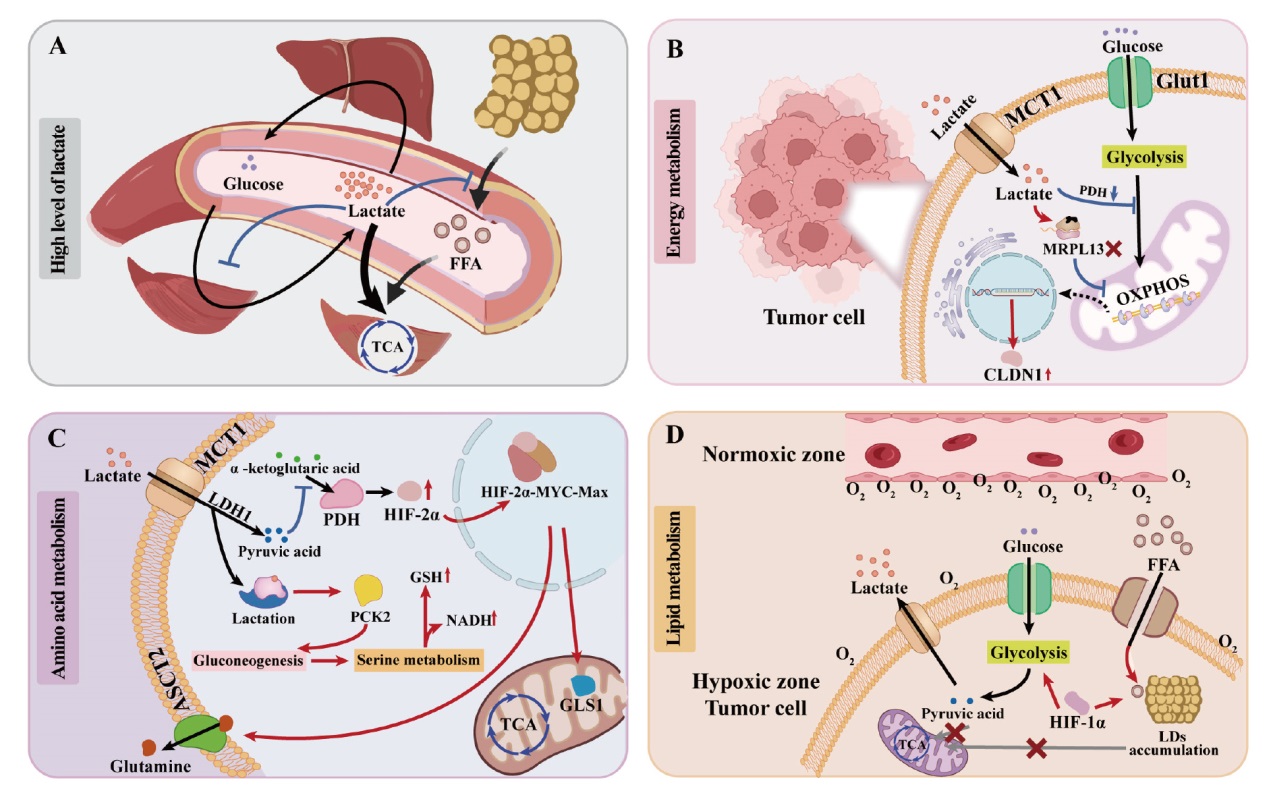
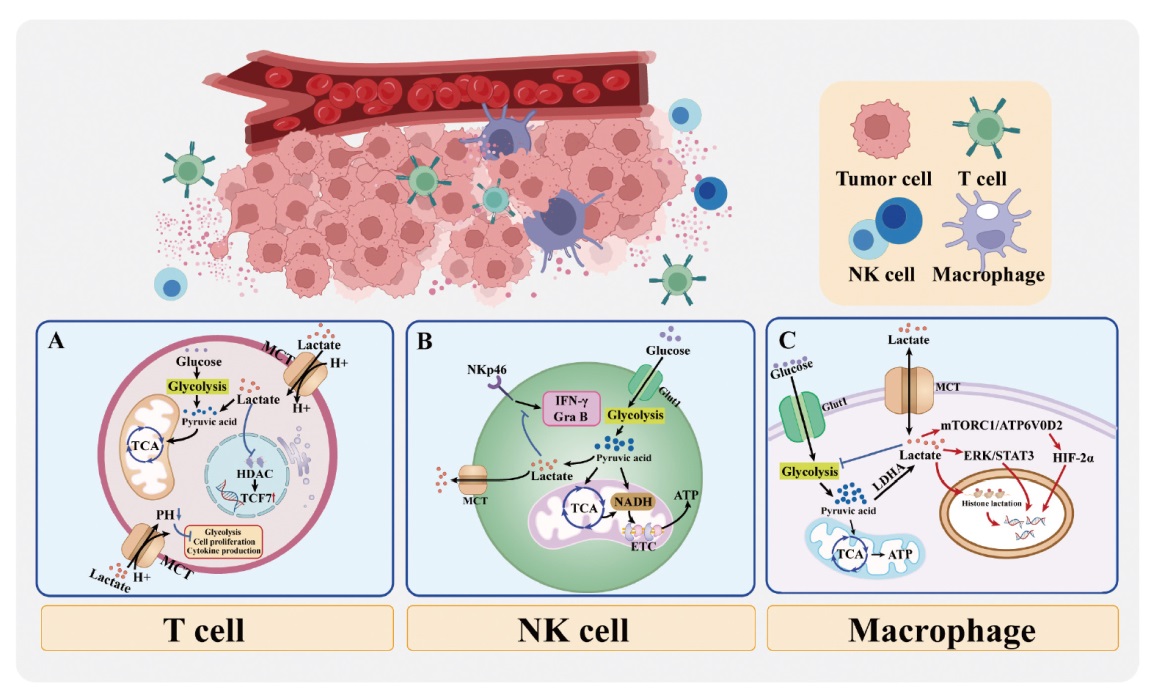
Fig. 2
Schematic diagram of lactate reshaping the immunosuppressive microenvironment A: Lactate inhibits the function of effector T cells; B: Lactate inhibits the activity of NK cells; C: Lactate promotes the polarization of macrophages. HDAC: Histone deacetylase; TCF7: Transcription factor 7; Gra B: Granzyme B."
| [1] | CAO W, QIN K, LI F, et al. Socioeconomic inequalities in cancer incidence and mortality: an analysis of GLOBOCAN 2022[J]. Chin Med J (Engl), 2024, 137(12): 1407-1413. |
| [2] | 郑荣寿, 陈茹, 韩冰峰, 等. 2022年中国恶性肿瘤流行情况分析[J]. 中华肿瘤杂志, 2024, 46(3): 221-231. |
| ZHENG R S, CHEN R, HAN B F, et al. Cancer incidence and mortality in China, 2022[J]. Chin J Oncol, 2024, 46(3): 221-231. | |
| [3] |
BROOKS G A. The tortuous path of lactate shuttle discovery: from cinders and boards to the lab and ICU[J]. J Sport Health Sci, 2020, 9(5): 446-460.
doi: S2095-2546(20)30019-3 pmid: 32444344 |
| [4] | WANG Q T, HU Y, WAN J L, et al. Lactate: a novel signaling molecule in synaptic plasticity and drug addiction[J]. Bioessays, 2019, 41(8): e1900008. |
| [5] |
EMHOFF C W, MESSONNIER L A, HORNING M A, et al. Gluconeogenesis and hepatic glycogenolysis during exercise at the lactate threshold[J]. J Appl Physiol (1985), 2013, 114(3): 297-306.
doi: 10.1152/japplphysiol.01202.2012 |
| [6] |
SHAO C, TANG S, YU S Q, et al. Genetic code expansion reveals site-specific lactylation in living cells reshapes protein functions[J]. Nat Commun, 2025, 16(1): 227.
doi: 10.1038/s41467-024-55165-2 |
| [7] |
HU Y, HE Z, LI Z, et al. Lactylation: the novel histone modification influence on gene expression, protein function, and disease[J]. Clin Epigenetics, 2024, 16(1): 72
doi: 10.1186/s13148-024-01682-2 pmid: 38812044 |
| [8] |
MAO Y, ZHANG J, ZHOU Q, et al. Hypoxia induces mitochondrial protein lactylation to limit oxidative phosphorylation[J]. Cell Res, 2024, 34(1): 13-30.
doi: 10.1038/s41422-023-00864-6 pmid: 38163844 |
| [9] |
LI L, CHEN K, WANG T, et al. Glis1 facilitates induction of pluripotency via an epigenome-metabolome-epigenome signalling cascade[J]. Nat Metab, 2020, 2(9): 882-92
doi: 10.1038/s42255-020-0267-9 |
| [10] |
ZHAO Q H, WANG Q, YAO Q H, et al. Nonenzymatic lysine D-lactylation induced by glyoxalase Ⅱ substrate SLG dampens inflammatory immune responses[J]. Cell Res, 2025, 35(2): 97-116.
doi: 10.1038/s41422-024-01060-w |
| [11] |
XU K, ZHANG K, WANG Y, et al. Comprehensive review of histone lactylation: structure, function, and therapeutic targets[J]. Biochem Pharmacol, 2024, 225: 116331.
doi: 10.1016/j.bcp.2024.116331 |
| [12] |
BROOKS G A. Cell-cell and intracellular lactate shuttles[J]. J Physiol, 2009, 587(Pt 23): 5591-5600.
doi: 10.1113/tjp.2009.587.issue-23 |
| [13] |
FAUBERT B, LI K Y, CAI L, et al. Lactate metabolism in human lung tumors[J]. Cell, 2017, 171(2): 358-371.e9.
doi: S0092-8674(17)31068-1 pmid: 28985563 |
| [14] |
WANG W T, ZHOU Y, LI W, et al. Claudins and hepatocellular carcinoma[J]. Biomed Pharmacother, 2024, 171: 116109.
doi: 10.1016/j.biopha.2023.116109 |
| [15] | WELCH D R. Tumor heterogeneity: a ‘contemporary concept’ founded on historical insights and predictions[J]. Cancer Res, 2016, 76(1): 4-6. |
| [16] |
ENRÍQUEZ J A, MITTELBRUNN M. Warburg effect reshapes tumor immunogenicity[J]. Cancer Res, 2024, 84(13): 2043-2045.
doi: 10.1158/0008-5472.CAN-24-1304 pmid: 38657107 |
| [17] |
CAPATINA A L, MALCOLM J R, STENNING J, et al. Hypoxia-induced epigenetic regulation of breast cancer progression and the tumour microenvironment[J]. Front Cell Dev Biol, 2024, 12: 1421629.
doi: 10.3389/fcell.2024.1421629 |
| [18] |
ZHANG G Q, XI C, JU N T, et al. Targeting glutamine metabolism exhibits anti-tumor effects in thyroid cancer[J]. J Endocrinol Invest, 2024, 47(8): 1953-1969.
doi: 10.1007/s40618-023-02294-y pmid: 38386265 |
| [19] |
XUAN D T M, WU C C, WANG W J, et al. Glutamine synthetase regulates the immune microenvironment and cancer development through the inflammatory pathway[J]. Int J Med Sci, 2023, 20(1): 35-49.
doi: 10.7150/ijms.75625 pmid: 36619229 |
| [20] |
MA Y, LING S K, LI Y, et al. Loss of heterozygosity for Kras G12D promotes malignant phenotype of pancreatic ductal adenocarcinoma by activating HIF-2α-c-myc-regulated glutamine metabolism[J]. Int J Mol Sci, 2022, 23(12): 6697.
doi: 10.3390/ijms23126697 |
| [21] |
WEN Q, HUANG M H, XIE J W, et al. lncRNA SYTL5-OT4 promotes vessel co-option by inhibiting the autophagic degradation of ASCT2[J]. Drug Resist Updat, 2023, 69: 100975.
doi: 10.1016/j.drup.2023.100975 |
| [22] |
HU H X, TAKANO N, XIANG L S, et al. Hypoxia-inducible factors enhance glutamate signaling in cancer cells[J]. Oncotarget, 2014, 5(19): 8853-8868.
doi: 10.18632/oncotarget.2593 pmid: 25326682 |
| [23] |
LAU A, BLENIS J, BURGOS-BARRAGAN G. Decoding serine metabolism: unveiling novel pathways for evolving cancer therapies[J]. Cancer Res, 2024, 84(8): 1191-1194.
doi: 10.1158/0008-5472.CAN-24-0541 pmid: 38364233 |
| [24] | LU Y X, ZHU J H, ZHANG Y X, et al. Lactylation-driven IGF2BP3-mediated serine metabolism reprogramming and RNA m6A-modification promotes lenvatinib resistance in HCC[J]. Adv Sci (Weinh), 2024, 11(46): e2401399. |
| [25] |
QIAO Z, LI Y, LI S M, et al. Hypoxia-induced SHMT2 protein lactylation facilitates glycolysis and stemness of esophageal cancer cells[J]. Mol Cell Biochem, 2024, 479(11): 3063-3076.
doi: 10.1007/s11010-023-04913-x pmid: 38175377 |
| [26] |
NAGANA GOWDA G A, LUSK J A, PASCUA V. Intracellular pyruvate-lactate-alanine cycling detected using real-time nuclear magnetic resonance spectroscopy of live cells and isolated mitochondria[J]. Magn Reson Chem, 2024, 62(2): 84-93.
doi: 10.1002/mrc.v62.2 |
| [27] |
GREEN C R, ALAEDDINE L M, WESSENDORF-RODRIGUEZ K A, et al. Impaired branched-chain amino acid (BCAA) catabolism during adipocyte differentiation decreases glycolytic flux[J]. J Biol Chem, 2024, 300(12): 108004.
doi: 10.1016/j.jbc.2024.108004 |
| [28] |
KONDO A, YAMAMOTO S, NAKAKI R, et al. Extracellular acidic pH activates the sterol regulatory element-binding protein 2 to promote tumor progression[J]. Cell Rep, 2017, 18(9): 2228-2242.
doi: S2211-1247(17)30171-7 pmid: 28249167 |
| [29] |
IPPOLITO L, COMITO G, PARRI M, et al. Lactate rewires lipid metabolism and sustains a metabolic-epigenetic axis in prostate cancer[J]. Cancer Res, 2022, 82(7): 1267-1282.
doi: 10.1158/0008-5472.CAN-21-0914 pmid: 35135811 |
| [30] |
CORBET C, BASTIEN E, SANTIAGO DE JESUS J P, et al. TGFβ2-induced formation of lipid droplets supports acidosis-driven EMT and the metastatic spreading of cancer cells[J]. Nat Commun, 2020, 11(1): 454.
doi: 10.1038/s41467-019-14262-3 pmid: 31974393 |
| [31] |
IPPOLITO L, COMITO G, PARRI M, et al. Lactate rewires lipid metabolism and sustains a metabolic-epigenetic axis in prostate cancer[J]. Cancer Res, 2022, 82(7): 1267-82.
doi: 10.1158/0008-5472.CAN-21-0914 pmid: 35135811 |
| [32] |
MENG Y, GUO D, LIN L, et al. Glycolytic enzyme PFKL governs lipolysis by promoting lipid droplet-mitochondria tethering to enhance β-oxidation and tumor cell proliferation[J]. Nat Metab, 2024, 6(6): 1092-107.
doi: 10.1038/s42255-024-01047-2 pmid: 38773347 |
| [33] |
WAN J, CHENG C, HU J J, et al. De novo NAD+ synthesis contributes to CD8+T cell metabolic fitness and antitumor function[J]. Cell Rep, 2023, 42(12): 113518.
doi: 10.1016/j.celrep.2023.113518 |
| [34] | TURNER L, VAN LE T N, CROSS E, et al. Single-cell NAD(H) levels predict clonal lymphocyte expansion dynamics[J]. Sci Immunol, 2024, 9(93): eadj7238. |
| [35] |
PAN W L, TSOKOS M G, SCHERLINGER M, et al. The PP2A regulatory subunit PPP2R2A controls NAD+ biosynthesis to regulate T cell subset differentiation in systemic autoimmunity[J]. Cell Rep, 2024, 43(7): 114379.
doi: 10.1016/j.celrep.2024.114379 |
| [36] | CHEN X Y, LIU L L, KANG S W, et al. The lactate dehydrogenase (LDH) isoenzyme spectrum enables optimally controlling T cell glycolysis and differentiation[J]. Sci Adv, 2023, 9(12): eadd9554. |
| [37] |
PERALTA R M, XIE B X, LONTOS K, et al. Dysfunction of exhausted T cells is enforced by MCT11-mediated lactate metabolism[J]. Nat Immunol, 2024, 25(12): 2297-2307.
doi: 10.1038/s41590-024-01999-3 pmid: 39516648 |
| [38] |
FANG Y, LIU W R, TANG Z, et al. Monocarboxylate transporter 4 inhibition potentiates hepatocellular carcinoma immunotherapy through enhancing T cell infiltration and immune attack[J]. Hepatology, 2023, 77(1): 109-123.
doi: 10.1002/hep.32348 |
| [39] |
BARBIERI L, VELIÇA P, GAMEIRO P A, et al. Lactate exposure shapes the metabolic and transcriptomic profile of CD8+T cells[J]. Front Immunol, 2023, 14: 1101433.
doi: 10.3389/fimmu.2023.1101433 |
| [40] | D’ARIA S, MAQUET C, LI S, et al. Expression of the monocarboxylate transporter MCT1 is required for virus-specific mouse CD8+T cell memory development[J]. Proc Natl Acad Sci USA, 2024, 121(13): e2306763121. |
| [41] |
SUKUMAR M, LIU J, JI Y, et al. Inhibiting glycolytic metabolism enhances CD8+T cell memory and antitumor function[J]. J Clin Invest, 2013, 123(10): 4479-4488.
doi: 10.1172/JCI69589 |
| [42] |
WATSON M J, VIGNALI P D A, MULLETT S J, et al. Metabolic support of tumour-infiltrating regulatory T cells by lactic acid[J]. Nature, 2021, 591(7851): 645-651.
doi: 10.1038/s41586-020-03045-2 |
| [43] |
KONDO M, KUMAGAI S, NISHIKAWA H. Metabolic advantages of regulatory T cells dictated by cancer cells[J]. Int Immunol, 2024, 36(2): 75-86.
doi: 10.1093/intimm/dxad035 |
| [44] |
ZHANG Y N, HUANG Y T, HONG Y, et al. Lactate acid promotes PD-1+ Tregs accumulation in the bone marrow with high tumor burden of acute myeloid leukemia[J]. Int Immunopharmacol, 2024, 130: 111765.
doi: 10.1016/j.intimp.2024.111765 |
| [45] |
TUOMELA K, LEVINGS M K. Acidity promotes the differentiation of immunosuppressive regulatory T cells[J]. Eur J Immunol, 2023, 53(6): 2350511.
doi: 10.1002/eji.v53.6 |
| [46] | RAO D S, STUNNENBERG J A, LACROIX R, et al. Acidity-mediated induction of FoxP3+ regulatory T cells[J]. Eur J Immunol, 2023, 53(6): e2250258. |
| [47] |
ZHANG Y T, XING M L, FANG H H, et al. Effects of lactate on metabolism and differentiation of CD4+T cells[J]. Mol Immunol, 2023, 154: 96-107.
doi: 10.1016/j.molimm.2022.12.015 |
| [48] |
ZHANG W H, YUAN S J, ZHANG Z P, et al. Regulating tumor cells to awaken T cell antitumor function and enhance melanoma immunotherapy[J]. Biomaterials, 2025, 316: 123034.
doi: 10.1016/j.biomaterials.2024.123034 |
| [49] |
MA J W, TANG L, TAN Y Y, et al. Lithium carbonate revitalizes tumor-reactive CD8+T cells by shunting lactic acid into mitochondria[J]. Nat Immunol, 2024, 25(3): 552-561.
doi: 10.1038/s41590-023-01738-0 |
| [50] | GE W L, MENG L D, CAO S J, et al. The SIX1/LDHA axis promotes lactate accumulation and leads to NK cell dysfunction in pancreatic cancer[J]. J Immunol Res, 2023, 2023: 6891636. |
| [51] |
BRAND A, SINGER K, KOEHL G E, et al. LDHA-associated lactic acid production blunts tumor immunosurveillance by T and NK cells[J]. Cell Metab, 2016, 24(5): 657-671.
doi: S1550-4131(16)30427-2 pmid: 27641098 |
| [52] | LUO Z H, HUANG X H, XU X Y, et al. Decreased LDHB expression in breast tumor cells causes NK cell activation and promotes tumor progression[J]. Cancer Biol Med, 2024, 21(6): 513-540. |
| [53] |
NETSKAR H, PFEFFERLE A, GOODRIDGE J P, et al. Pan-cancer profiling of tumor-infiltrating natural killer cells through transcriptional reference mapping[J]. Nat Immunol, 2024, 25(8): 1445-1459.
doi: 10.1038/s41590-024-01884-z pmid: 38956379 |
| [54] |
GLASNER A, LEVI A, ENK J, et al. NKp46 receptor-mediated interferon-γ production by natural killer cells increases fibronectin 1 to alter tumor architecture and control metastasis[J]. Immunity, 2018, 48(1): 107-119.e4.
doi: S1074-7613(17)30536-8 pmid: 29329948 |
| [55] |
HARMON C, ROBINSON M W, HAND F, et al. Lactate-mediated acidification of tumor microenvironment induces apoptosis of liver-resident NK cells in colorectal liver metastasis[J]. Cancer Immunol Res, 2019, 7(2): 335-346.
doi: 10.1158/2326-6066.CIR-18-0481 pmid: 30563827 |
| [56] |
DODARD G, TATA A, ERICK T K, et al. Inflammation-induced lactate leads to rapid loss of hepatic tissue-resident NK cells[J]. Cell Rep, 2020, 32(1): 107855
doi: 10.1016/j.celrep.2020.107855 |
| [57] |
ZHANG Y P, ZHONG F, LIU L. Single-cell transcriptional atlas of tumor-associated macrophages in breast cancer[J]. Breast Cancer Res, 2024, 26(1): 129.
doi: 10.1186/s13058-024-01887-6 pmid: 39232806 |
| [58] |
LIU N, LUO J, KUANG D, et al. Lactate inhibits ATP6V0d2 expression in tumor-associated macrophages to promote HIF-2α-mediated tumor progression[J]. J Clin Invest, 2019, 129(2): 631-646.
doi: 10.1172/JCI123027 pmid: 30431439 |
| [59] |
CAI Z N, LI W, HAGER S, et al. Targeting PHGDH reverses the immunosuppressive phenotype of tumor-associated macrophages through α-ketoglutarate and mTORC1 signaling[J]. Cell Mol Immunol, 2024, 21(5): 448-465.
doi: 10.1038/s41423-024-01134-0 pmid: 38409249 |
| [60] | VADEVOO S M P, GUNASSEKARAN G R, LEE C, et al. The macrophage odorant receptor Olfr78 mediates the lactate-induced M2 phenotype of tumor-associated macrophages[J]. Proc Natl Acad Sci USA, 2021, 118(37): e2102434118. |
| [61] |
KHAN F, LIN Y Y, ALI H B, et al. Lactate dehydrogenase A regulates tumor-macrophage symbiosis to promote glioblastoma progression[J]. Nat Commun, 2024, 15(1): 1987.
doi: 10.1038/s41467-024-46193-z pmid: 38443336 |
| [62] |
LIU M Y, REN Y, ZHOU Z J, et al. The crosstalk between macrophages and cancer cells potentiates pancreatic cancer cachexia[J]. Cancer Cell, 2024, 42(5): 885-903.e4.
doi: 10.1016/j.ccell.2024.03.009 pmid: 38608702 |
| [63] |
JIANG R, REN W J, WANG L Y, et al. Targeting lactate: an emerging strategy for macrophage regulation in chronic inflammation and cancer[J]. Biomolecules, 2024, 14(10): 1202.
doi: 10.3390/biom14101202 |
| [64] |
SHU M, LU D C, ZHU Z Y, et al. Insight into the roles of lactylation in macrophages: functions and clinical implications[J]. Clin Sci (Lond), 2025, 139(2): 151-169.
doi: 10.1042/CS20242737 |
| [65] |
SUN J R, FENG Q M, HE Y, et al. Lactate activates CCL18 expression via H3K18 lactylation in macrophages to promote tumorigenesis of ovarian cancer[J]. Acta Biochim Biophys Sin (Shanghai), 2024, 56(9): 1373-1386.
doi: 10.3724/abbs.2024111 |
| [66] |
SACHA M, FAUCON L, HAMON E, et al. Ex vivo transdermal absorption of a liposome formulation of diclofenac[J]. Biomed Pharmacother, 2019, 111: 785-790.
doi: 10.1016/j.biopha.2018.12.079 |
| [67] | LI X L, JIANG C, WANG Q H, et al. A “valve-closing” starvation strategy for amplification of tumor-specific chemotherapy[J]. Adv Sci (Weinh), 2022, 9(8): e2104671. |
| [68] |
BRESINSKY M, GOEPFERICH A. Control of biomedical nanoparticle distribution and drug release in vivo by complex particle design strategies[J]. Eur J Pharm Biopharm, 2025, 208: 114634.
doi: 10.1016/j.ejpb.2025.114634 |
| [69] |
DING X L, LIU M D, CHENG Q, et al. Multifunctional liquid metal-based nanoparticles with glycolysis and mitochondrial metabolism inhibition for tumor photothermal therapy[J]. Biomaterials, 2022, 281: 121369.
doi: 10.1016/j.biomaterials.2022.121369 |
| [70] |
DUAN R X, XU Y Y, ZENG X M, et al. Uncovering the metabolic origin of aspartate for tumor growth using an integrated molecular deactivator[J]. Nano Lett, 2021, 21(1): 778-784.
doi: 10.1021/acs.nanolett.0c04520 pmid: 33301328 |
| [71] | WANG D W, REN X H, MA Y J, et al. Dual-template epitope imprinted nanoparticles for anti-glycolytic tumor-targeted treatment[J]. J Colloid Interface Sci, 2025, 683(Pt 2): 890-905. |
| [72] |
FARAH C, NEVEU M A, BOUZIN C, et al. Hyperpolarized 13C-pyruvate to assess response to anti-PD1 immune checkpoint inhibition in YUMMER 1.7 melanoma xenografts[J]. Int J Mol Sci, 2023, 24(3): 2499.
doi: 10.3390/ijms24032499 |
| [73] |
ZHOU J R, HU Y P, CAO Y H, et al. A Lactate-Depleting metal organic framework-based nanocatalyst reinforces intratumoral T cell response to boost anti-PD1 immunotherapy[J]. J Colloid Interface Sci, 2024, 660: 869-884.
doi: 10.1016/j.jcis.2024.01.129 |
| [74] |
WANG H J, WU C H, TONG X W, et al. A biomimetic metal-organic framework nanosystem modulates immunosuppressive tumor microenvironment metabolism to amplify immunotherapy[J]. J Control Release, 2023, 353: 727-737.
doi: 10.1016/j.jconrel.2022.11.054 |
| [75] |
SOMCHOB B, PROMPHET N, RODTHONGKUM N, et al. Zwitterionic hydrogel for preserving stability and activity of oxidase enzyme for electrochemical biosensor[J]. Talanta, 2024, 270: 125510.
doi: 10.1016/j.talanta.2023.125510 |
| [76] |
CHOI H, YEO M, KANG Y J, et al. Lactate oxidase/catalase-displaying nanoparticles efficiently consume lactate in the tumor microenvironment to effectively suppress tumor growth[J]. J Nanobiotechnology, 2023, 21(1): 5.
doi: 10.1186/s12951-022-01762-6 |
| [77] | ZHAO S F, HOU J N, DENG L, et al. Lactate-modulating nanozyme-mediated mitochondrial respiration block for tumor immunosuppression remodeling[J]. Angew Chem Int Ed, 2025, 64(17): e202422203. |
| [78] |
ZHAO S F, LI H H, LIU R Y, et al. Nitrogen-centered lactate oxidase nanozyme for tumor lactate modulation and microenvironment remodeling[J]. J Am Chem Soc, 2023, 145(18): 10322-10332.
doi: 10.1021/jacs.3c02005 |
| [79] |
NIE T Q, FANG Y F, ZHANG R H, et al. Self-healable and pH-responsive spermidine/ferrous ion complexed hydrogel co-loaded with CA inhibitor and glucose oxidase for combined cancer immunotherapy through triple ferroptosis mechanism[J]. Bioact Mater, 2025, 47: 51-63.
doi: 10.1016/j.bioactmat.2025.01.005 pmid: 39877156 |
| [80] | MAO L Z, XARPIDIN B, SHI R, et al. Natural enzyme-loaded polymeric stealth coating-armed engineered probiotics by disrupting tumor lactate homeostasis to synergistic metabolism-immuno-enzyme dynamic therapy[J]. Adv Sci (Weinh), 2025, 12(16): e2417172. |
| [1] | WU Jiachen, HE Lina, TANG Xinru, TANG Shuang. The research on construction of the spontaneous prostate tumor and breast cancer model of Ptenfl/fl;Trp53fl/fl;Pbsn-iCre+ transgenic mouse [J]. China Oncology, 2025, 35(8): 769-775. |
| [2] | LIU Chun, CHEN Guoxin, LI Mengtian, ZHAO Yu, MA Zhongren, ZHANG Haixia. Research progress and prospects of virus-like particles in tumor therapy [J]. China Oncology, 2025, 35(6): 585-591. |
| [3] | GONG Weihua, CHEN Lan, ZHAO Kun, KE Zhui, XU Qing, GUO Xianling. Mechanism of telomerase inhibitor BIBR1532 combined with autophagy inhibitor CQ in suppressing survival of melanoma cells [J]. China Oncology, 2025, 35(5): 431-439. |
| [4] | YING Leilei, LI Kening, CHEN Chao, WANG Ying, HUANG Haozhe, WANG Biao, LI Wentao, HE Xinhong. Impact of tumor diameter on post-radiofrequency ablation survival and local progression risk in patients with colorectal cancer lung metastasis [J]. China Oncology, 2025, 35(5): 449-456. |
| [5] | HU Wei, REN Xiaomeng, WANG Yang, ZHAO Peiqing, CAO Kai. TIPE regulates glucometabolic reprogramming by modulating LDHA expression in triple-negative breast cancer [J]. China Oncology, 2025, 35(4): 386-393. |
| [6] | ZENG Dandan, LUO Wenfeng, YE Jiazhou, LIN Yan, LIANG Rong. Research progress and prospect of histone lactylation in digestive system tumors [J]. China Oncology, 2025, 35(4): 424-430. |
| [7] | AN Tianqi, TIAN Jianhui, ZHOU Yiyang, LUO Bin, QUE Zujun, LIU Yao, YU Pan, ZHAO Ruihua, YANG Yun. Research progress on treatment of pleural effusion related to immune checkpoint inhibitors [J]. China Oncology, 2025, 35(3): 333-338. |
| [8] | CAI Shuyue, XIE Quan, ZHOU Yuxuan, LIU Qingzhu, QIU Ling, LIN Jianguo. Latest progress and prospect of NRP-1 targeted molecular probes for breast cancer diagnosis [J]. China Oncology, 2025, 35(2): 249-254. |
| [9] | ZHAO Jiaxuan, WANG Yixuan, TIAN Gaohui, SHI Jiangzhou, ZHANG Tongcun. A study on optimization of the CAR-γδ T cell manufacturing process [J]. China Oncology, 2025, 35(11): 1019-1031. |
| [10] | SUN Dawei, YU Jinyu, ZHANG Xin, ZHAO Songbo, ZHANG Xianzheng. A study on gallic acid enhancing the anti-solid tumor function of CAR-T cells [J]. China Oncology, 2025, 35(11): 1032-1040. |
| [11] | LIU Miaomiao, HUANG Yushan, YANG Guozi, TAI Panpan, CHEN Xiao, LIU Min, PAN Zhenyu. Phase Ⅰ study of intrathecal pemetrexed combined with programmed death-1 inhibitor for leptomeningeal metastases from solid tumors [J]. China Oncology, 2025, 35(11): 1041-1048. |
| [12] | LIU Hao, SU Junjie, XIN Shiyong. Mechanism study of MYC promoting proliferation and metastasis in prostate cancer by targeting CD47 [J]. China Oncology, 2025, 35(11): 987-1000. |
| [13] | YU Xue, SHEN Tianhao, ZHOU Cheng, LIU Yu, JIANG Tinghui, LI Wei, ZHU Yongqiang, LIU Yan. Research progress and prospects on the mechanisms of circulating tumor cells in the invasion and metastasis of cholangiocarcinoma [J]. China Oncology, 2025, 35(10): 952-958. |
| [14] | Multiple Primary and Unknown Primary Tumors Special Committee of China Anti-Cancer Association , Shanghai Anti Cancer Association Multiple Primary and Unknown Primary Tumor Special Committee , Lymphocytic Disease Group, Hematology Branch, Chinese Medical Association , Rare Disease Group, Hematology Branch, Chinese Medical Association . Expert consensus on diagnosis and treatment of hematologic malignancies with solid tumors (2025 version) [J]. China Oncology, 2025, 35(10): 968-986. |
| [15] | WANG Zifei, DING Yahui, LI Yan, LUAN Xin, TANG Min. Application of 3D bioprinting in cancer research and tissue engineering [J]. China Oncology, 2024, 34(9): 814-826. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
沪ICP备12009617
Powered by Beijing Magtech Co. Ltd
