Welcome to China Oncology,
China Oncology ›› 2024, Vol. 34 ›› Issue (7): 639-649.doi: 10.19401/j.cnki.1007-3639.2024.07.003
• Article • Previous Articles Next Articles
WANG Yixuan1( ), YU Miao1, ZHAO Jiaxuan1, ZHAO Fenfang2, ZENG Yi2, WANG Youyong2, ZHU Haichuan2, ZHANG Tongcun1,2, SHI Jiangzhou2(
), YU Miao1, ZHAO Jiaxuan1, ZHAO Fenfang2, ZENG Yi2, WANG Youyong2, ZHU Haichuan2, ZHANG Tongcun1,2, SHI Jiangzhou2( )
)
Received:2023-11-25
Revised:2024-02-22
Online:2024-07-30
Published:2024-08-08
Contact:
SHI Jiangzhou
Share article
CLC Number:
WANG Yixuan, YU Miao, ZHAO Jiaxuan, ZHAO Fenfang, ZENG Yi, WANG Youyong, ZHU Haichuan, ZHANG Tongcun, SHI Jiangzhou. Optimization study of CAR-T cell expansion targeting CD99[J]. China Oncology, 2024, 34(7): 639-649.
Tab. 1
shRNA sequence information targeting CD99"
| shRNA | Sequence |
|---|---|
| shRNA-1 | Forward: 5’-CGGATGGTGGTTTCGATTTATCTCGAGATAAATCGAAACCACCATCCGTTTTTT-3’ |
| Reverse: 5’-AAAAAACGGATGGTGGTTTCGATTTATCTCGAGATAAATCGAAACCACCATCCG-3’ | |
| shRNA-2 | Forward: 5’-CCAGCTGTTCAGCGTACTCTTCTCGAGAAGAGTACGCTGAACAGCTGGTTTTTT-3’ |
| Reverse: 5’-AAAAAACCAGCTGTTCAGCGTACTCTTCTCGAGAAGAGTACGCTGAACAGCTGG-3’ | |
| shRNA-control | Forward: 5’-CAACACAGATGATAGAGCACCAATTGGTGCTCTATCATCTGTGTTGTTTTT-3’ |
| Reverse: 5’-AAAAACAACACAGATGATAGAGCACCAATTGGTGCTCTATCATCTGTGTTG-3’ |
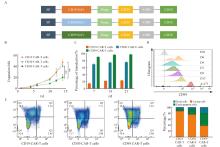
Fig. 1
The CD99 CAR-T cells exhibit proliferation challenges A: Structure diagram of CD19-CAR, CD30-CAR and CD99-CAR; B:Fold expansion of CD19 CAR-T cells, CD30 CAR-T cells and CD99 CAR-T cells; C: Transduction efficiency of CD19 CAR-T cells, CD30 CAR-T cells and CD99 CAR-T cells; D: Expression of CD99 protein on T cells and A-673 cells; E: The flow apoptosis diagram of CD19 CAR-T cells, CD30 CAR-T cells and CD99 CAR-T cells. **: P<0.01, compared with CD99 CAR-T cells."
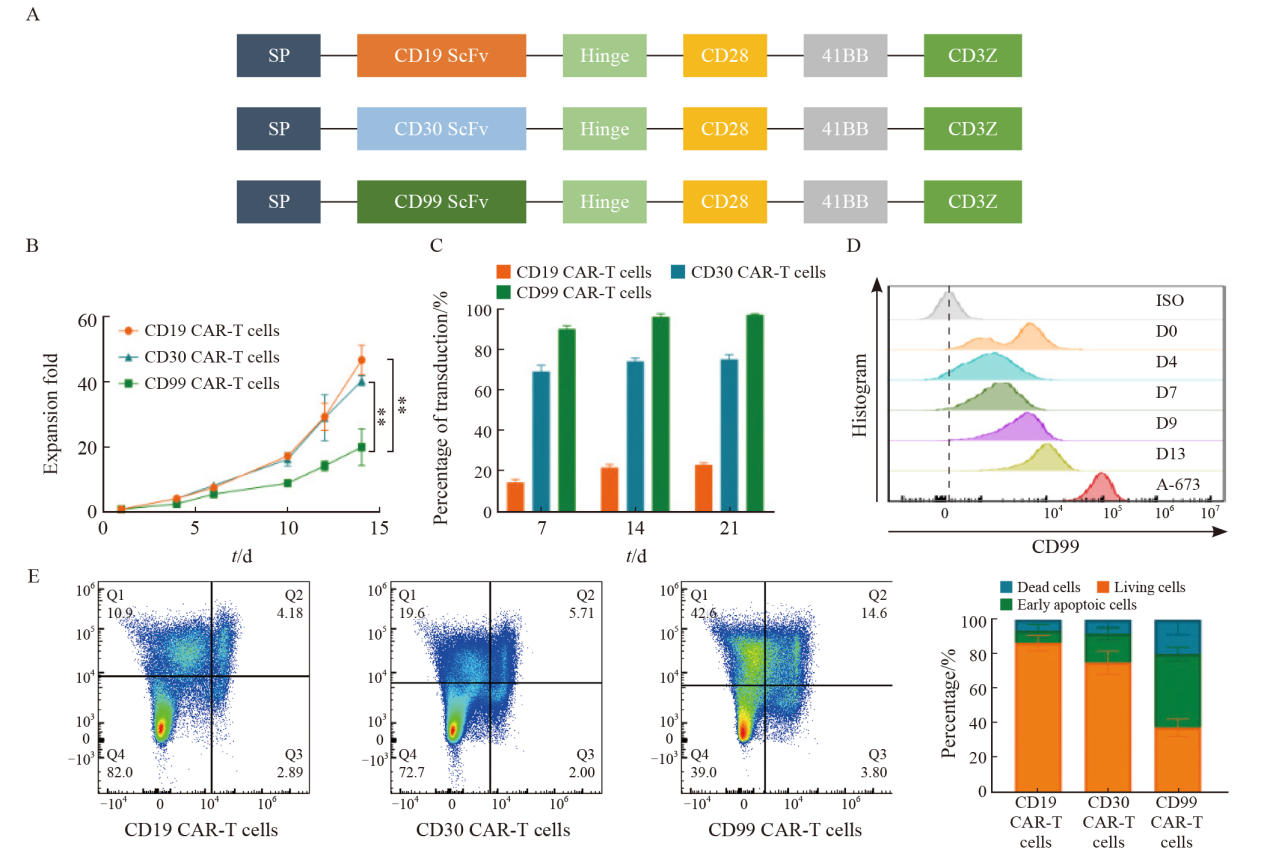
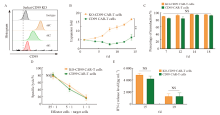
Fig. 2
Enhancement of CD99 CAR-T cell expansion by shRNA technology A: Knockdown the expression of CD99 protein on the cell surface of different shRNA; B: Proliferation of CD99 CAR-T cells and KO-CD99 CAR-T cells; C: Transduction efficiency of CD99 CAR-T cells and KO-CD99 CAR-T cells; D: Killing effect of CD99 CAR-T cells and KO-CD99 CAR-T cells; E: IFN-γ release level of CD99 CAR-T cells and KO-CD99 CAR-T cells; **: P<0.01, compared with CD99 CAR-T cells; NS: No significance."
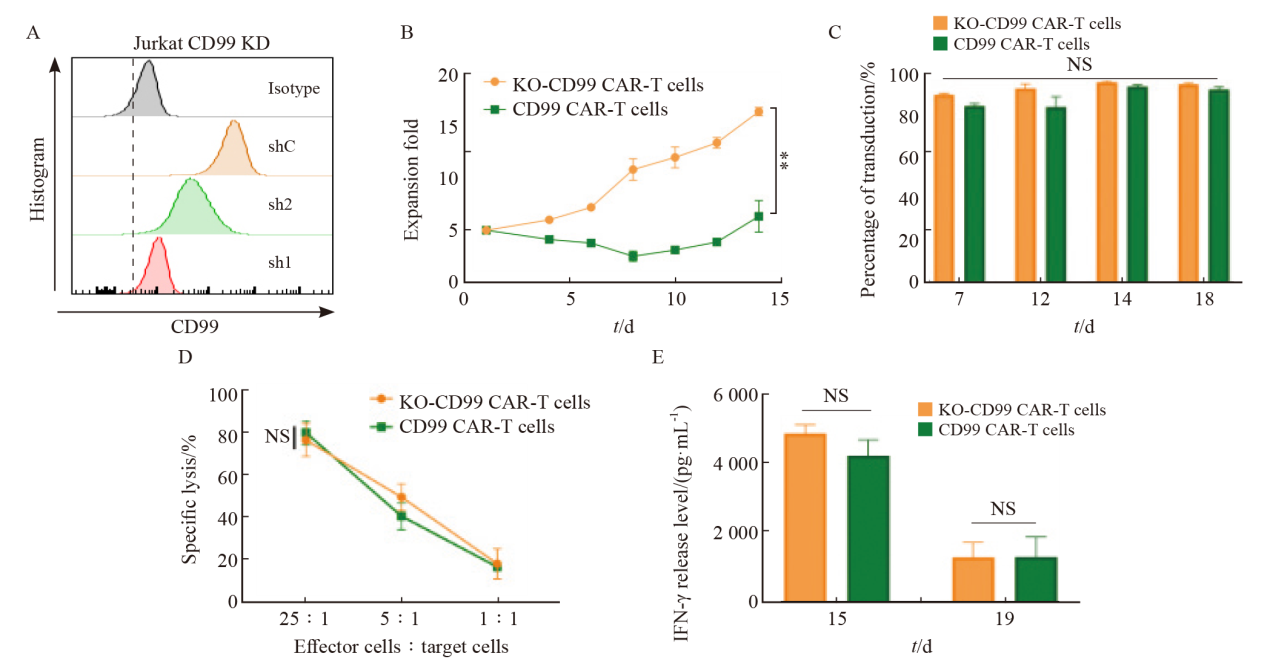
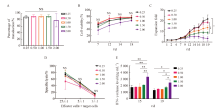
Fig. 3
Effects of different MOIs on sh-CD99 CAR-T cell transfection A: Transfection efficiency of KO-CD99 CAR-T cells in each MOI; B: Cell viability of KO-CD99 CAR-T cells obtained from different MOI; C: Fold expansion of KO-CD99 CAR-T cells obtained from different MOI; D: Killing effect of KO-CD99 CAR-T cells obtained from different MOI; E: IFN- γ release level of KO-CD99 CAR-T cells obtained from different MOI. *: P<0.05; **: P<0.01; NS: No significance."
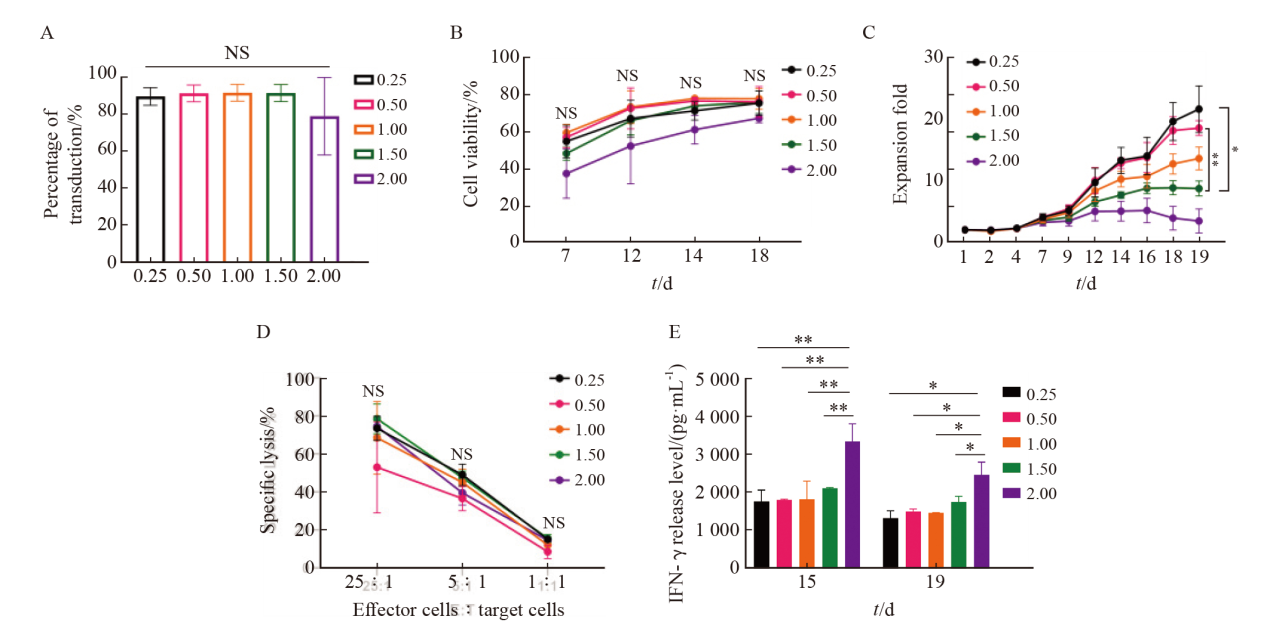
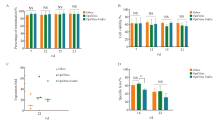
Fig. 4
Effect of different culture media on KO-CD99 CAR-T cells A: Transfection efficiency of KO-CD99 CAR-T cells in different medium; B: Cell viability of KO-CD99 CAR-T cells in different medium; C: Fold expansion of KO-CD99 CAR-T cells in different medium; D: Killing effect of KO-CD99 CAR-T cells in different medium. **: P<0.01, compared with OptiVitro; NS: No significance."
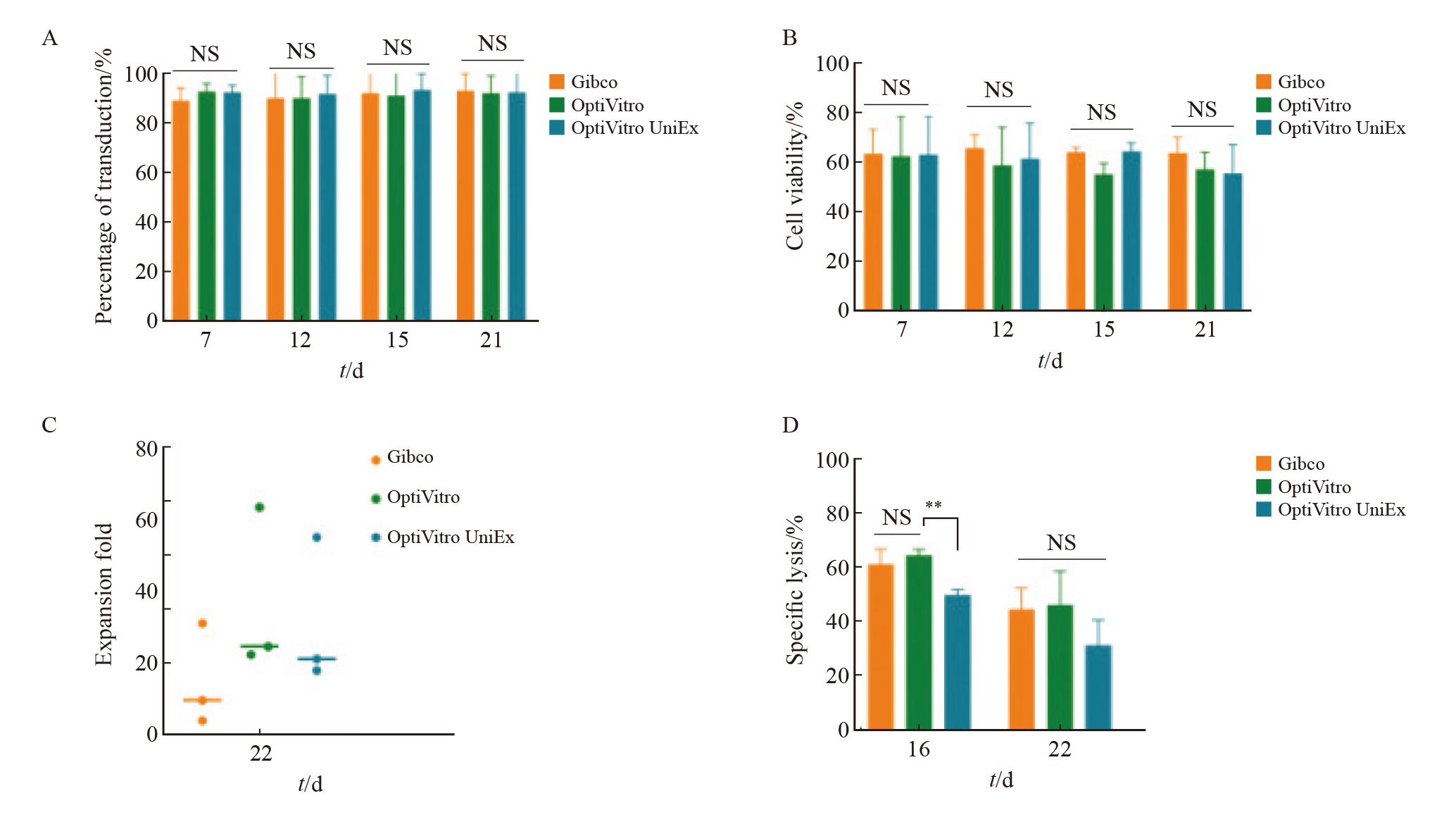
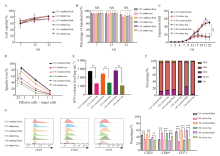
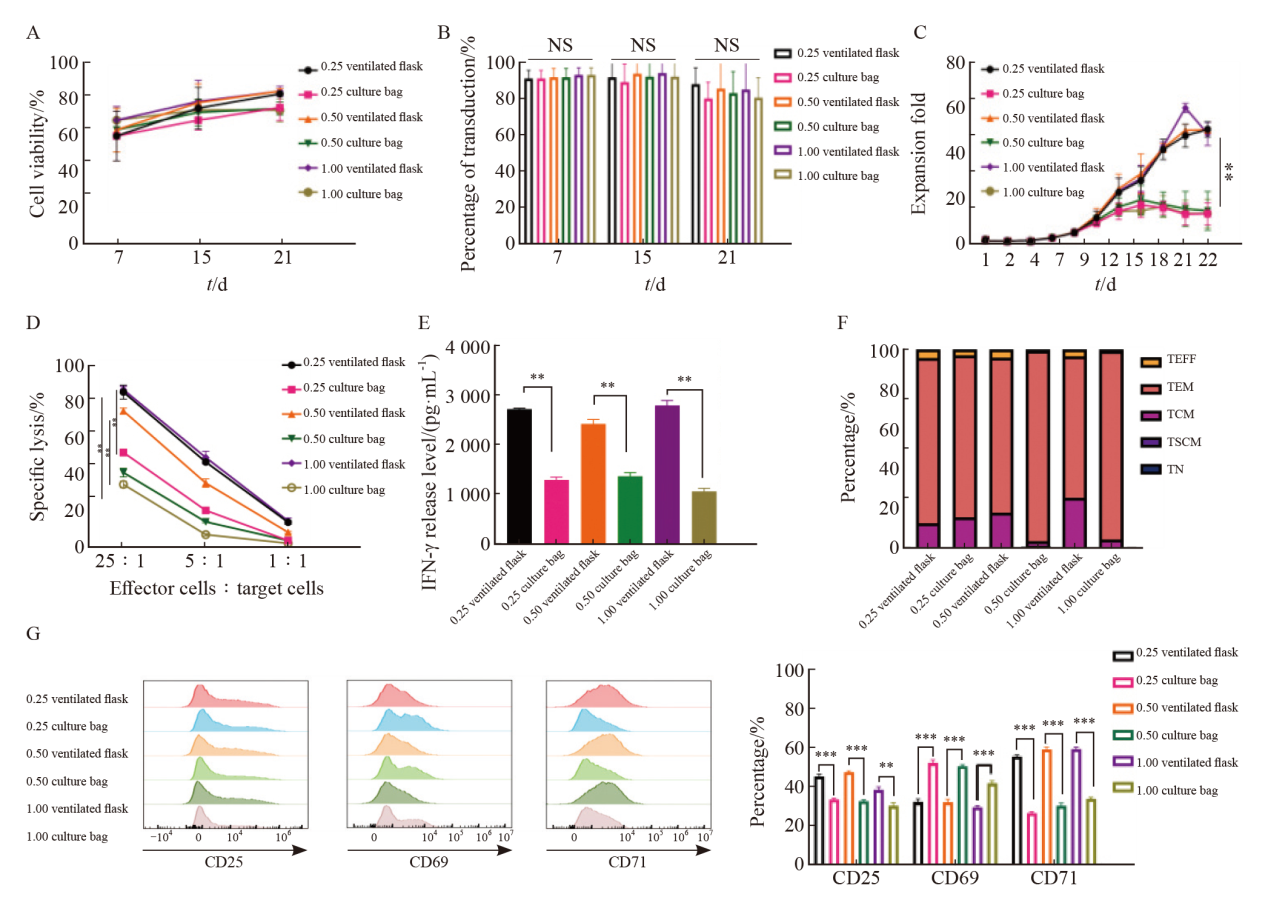
Fig. 5
Effects of different MOIs and culture vessels on KO-CD99 CAR-T cells A: Transfection efficiency of KO-CD99 CAR-T cells in each MOI and different culture containers; B: Cell viability of KO-CD99 CAR-T cells obtained from different MOI and different culture containers; C: Fold expansion of KO-CD99 CAR-T cells obtained from different MOI and different culture containers; D: Killing effect of KO-CD99 CAR-T cells obtained from different MOI and different culture container; E: IFN-γ release level of KO-CD99 CAR-T cells obtained from different MOI and different culture containers; F: Cluster of differentiation of KO-CD99 CAR-T cells obtained from different MOI and different culture containers; G: The expression level of CD25, CD69 and CD71 on KO-CD99 CAR-T cells obtained from different MOI and different culture containers. TEFF: Effector T cell; TEM: Effector memory T cell; TCM: Central memory T cell; TSCM: Stem cell memory T cell; TN: Naive T cell. **: P<0.01; ***: P<0.001."
| [1] | ZHANG X, LI J J, LU P H. Advances in the development of chimeric antigen receptor-T-cell therapy in B-cell acute lymphoblastic leukemia[J]. Chin Med J, 2020, 133(4): 474-482. |
| [2] |
PARK J H, GEYER M B, BRENTJENS R J. CD19-targeted CAR T-cell therapeutics for hematologic malignancies: interpreting clinical outcomes to date[J]. Blood, 2016, 127(26): 3312-3320.
doi: 10.1182/blood-2016-02-629063 pmid: 27207800 |
| [3] |
MAROFI F, MOTAVALLI R, SAFONOV V A, et al. CAR T cells in solid tumors: challenges and opportunities[J]. Stem Cell Res Ther, 2021, 12(1): 81.
doi: 10.1186/s13287-020-02128-1 pmid: 33494834 |
| [4] |
WAGNER J, WICKMAN E, DERENZO C, et al. CAR T cell therapy for solid tumors: bright future or dark reality?[J]. Mol Ther, 2020, 28(11): 2320-2339.
doi: 10.1016/j.ymthe.2020.09.015 pmid: 32979309 |
| [5] | RIGGI N, CIRONI L, PROVERO P, et al. Development of Ewing sarcoma from primary bone marrow-derived mesenchymal progenitor cells[J]. Cancer Res, 2005, 65(24): 11459-11468. |
| [6] | RIGGI N, SUVÀ M L, SUVÀ D, et al. EWS-FLI-1 expression triggers a Ewing sarcoma initiation program in primary human mesenchymal stem cells[J]. Cancer Res, 2008, 68(7): 2176-2185. |
| [7] | DESAI S S, JAMBHEKAR N A. Pathology of Ewing sarcoma/PNET: current opinion and emerging concepts[J]. Indian J Orthop, 2010, 44(4): 363-368. |
| [8] | KALLEN M E, HORNICK J L. The 2020 WHO classification[J]. Am J Surg Pathol, 2020, 45(1): e1-e23. |
| [9] | VAN MATER D, WAGNER L. Management of recurrent Ewing sarcoma: challenges and approaches[J]. Onco Targets Ther, 2019, 12: 2279-2288. |
| [10] |
BALDAUF M C, ORTH M F, DALLMAYER M, et al. Robust diagnosis of Ewing sarcoma by immunohistochemical detection of super-enhancer-driven EWSR1-ETS targets[J]. Oncotarget, 2018, 9(2): 1587-1601.
doi: 10.18632/oncotarget.20098 pmid: 29416716 |
| [11] |
ROCCHI A, MANARA M C, SCIANDRA M, et al. CD99 inhibits neural differentiation of human Ewing sarcoma cells and thereby contributes to oncogenesis[J]. J Clin Invest, 2010, 120(3): 668-680.
doi: 10.1172/JCI36667 pmid: 20197622 |
| [12] |
ZHANG S K, GU C J, HUANG L F, et al. The third-generation anti-CD30 CAR T-cells specifically homing to the tumor and mediating powerful antitumor activity[J]. Sci Rep, 2022, 12(1): 10488.
doi: 10.1038/s41598-022-14523-0 pmid: 35729339 |
| [13] |
ELSALLAB M, LEVINE B L, WAYNE A S, et al. CAR T-cell product performance in haematological malignancies before and after marketing authorisation[J]. Lancet Oncol, 2020, 21(2): e104-e116.
doi: 10.1016/S1470-2045(19)30729-6 pmid: 32007196 |
| [14] | FEINS S, KONG W M, WILLIAMS E F, et al. An introduction to chimeric antigen receptor (CAR) T-cell immunotherapy for human cancer[J]. Am J Hematol, 2019, 94(S1): S3-S9. |
| [15] |
AHMED N, SALSMAN V S, YVON E, et al. Immunotherapy for osteosarcoma: genetic modification of T cells overcomes low levels of tumor antigen expression[J]. Mol Ther, 2009, 17(10): 1779-1787.
doi: 10.1038/mt.2009.133 pmid: 19532139 |
| [16] |
ANDERS K, BLANKENSTEIN T. Molecular pathways: comparing the effects of drugs and T cells to effectively target oncogenes[J]. Clin Cancer Res, 2013, 19(2): 320-326.
doi: 10.1158/1078-0432.CCR-12-3017 pmid: 23197254 |
| [17] |
SCIANDRA M, MARINO M T, MANARA M C, et al. CD99 drives terminal differentiation of osteosarcoma cells by acting as a spatial regulator of ERK 1/2[J]. J Bone Miner Res, 2014, 29(5): 1295-1309.
doi: 10.1002/jbmr.2141 pmid: 24677094 |
| [18] | BYUN H J, HONG I K, KIM E, et al. A splice variant of CD99 increases motility and MMP-9 expression of human breast cancer cells through the AKT-, ERK-, and JNK-dependent AP-1 activation signaling pathways[J]. J Biol Chem, 2006, 281(46): 34833-34847. |
| [19] | BACCAR A, FERCHICHI I, TROUDI W, et al. CD99 and HLA-Ⅱ immunostaining in breast cancer tissue and their correlation with lymph node metastasis[J]. Dis Markers, 2013, 34(5): 363-371. |
| [20] |
GOTO A, NIKI T, TERADO Y, et al. Prevalence of CD99 protein expression in pancreatic endocrine tumours (PETs)[J]. Histopathology, 2004, 45(4): 384-392.
pmid: 15469477 |
| [21] | CARDOSO L C, SOARES R D S, LAURENTINO T S, et al. CD99 expression in glioblastoma molecular subtypes and role in migration and invasion[J]. Int J Mol Sci, 2019, 20(5): 1137. |
| [22] |
PASELLO M, MANARA M C, SCOTLANDI K. CD99 at the crossroads of physiology and pathology[J]. J Cell Commun Signal, 2018, 12(1): 55-68.
doi: 10.1007/s12079-017-0445-z pmid: 29305692 |
| [23] |
SCOTLANDI K, BALDINI N, CERISANO V, et al. CD99 engagement: an effective therapeutic strategy for Ewing tumors[J]. Cancer Res, 2000, 60(18): 5134-5142.
pmid: 11016640 |
| [24] | SHI J Z, ZHANG Z J, CEN H, et al. CAR T cells targeting CD99 as an approach to eradicate T-cell acute lymphoblastic leukemia without normal blood cells toxicity[J]. J Hematol Oncol, 2021, 14(1): 162. |
| [25] |
JIN J J, SABATINO M, SOMERVILLE R, et al. Simplified method of the growth of human tumor infiltrating lymphocytes in gas-permeable flasks to numbers needed for patient treatment[J]. J Immunother, 2012, 35(3): 283-292.
doi: 10.1097/CJI.0b013e31824e801f pmid: 22421946 |
| [26] | BAJGAIN P, MUCHARLA R, WILSON J, et al. Optimizing the production of suspension cells using the G-Rex “M” series[J]. Mol Ther Methods Clin Dev, 2014, 1: 14015. |
| [27] |
GAGLIARDI C, KHALIL M, FOSTER A E. Streamlined production of genetically modified T cells with activation, transduction and expansion in closed-system G-Rex bioreactors[J]. Cytotherapy, 2019, 21(12): 1246-1257.
doi: S1465-3249(19)30867-9 pmid: 31837737 |
| [28] |
LUDWIG J, HIRSCHEL M. Methods and process optimization for large-scale CAR T expansion using the G-rex cell culture platform[J]. Methods Mol Biol, 2020, 2086: 165-177.
doi: 10.1007/978-1-0716-0146-4_12 pmid: 31707675 |
| [29] | GARCIA-APONTE O F, HERWIG C, KOZMA B. Lymphocyte expansion in bioreactors: upgrading adoptive cell therapy[J]. J Biol Eng, 2021, 15(1): 13. |
| [30] |
SALMERÓN A, BORROTO A, FRESNO M, et al. Transferrin receptor induces tyrosine phosphorylation in T cells and is physically associated with the TCR zeta-chain[J]. J Immunol, 1995, 154(4): 1675-1683.
pmid: 7836751 |
| [31] |
LUM J B, INFANTE A J, MAKKER D M, et al. Transferrin synthesis by inducer T lymphocytes[J]. J Clin Invest, 1986, 77(3): 841-849.
pmid: 3005367 |
| [32] |
MOTAMEDI M, XU L, ELAHI S. Correlation of transferrin receptor (CD71) with Ki67 expression on stimulated human and mouse T cells: the kinetics of expression of T cell activation markers[J]. J Immunol Methods, 2016, 437: 43-52.
doi: 10.1016/j.jim.2016.08.002 pmid: 27555239 |
| [33] | NGUYEN X D, EICHLER H, DUGRILLON A, et al. Flow cytometric analysis of T cell proliferation in a mixed lymphocyte reaction with dendritic cells[J]. J Immunol Methods, 2003, 275(1/2): 57-68. |
| [34] |
SCHWAB L, MICHEL G, BEIN G, et al. CD71 surface analysis of T cells: a simple alternative for extracorporeal photopheresis quality control[J]. Vox Sang, 2020, 115(1): 81-93.
doi: 10.1111/vox.12850 pmid: 31680273 |
| [35] | CHEN C, GU Y M, ZHANG F, et al. Construction of PD1/CD28 chimeric-switch receptor enhances anti-tumor ability of c-Met CAR-T in gastric cancer[J]. Oncoimmunology, 2021, 10(1): 1901434. |
| [36] |
RATHMELL J C, VANDER HEIDEN M G, HARRIS M H, et al. In the absence of extrinsic signals, nutrient utilization by lymphocytes is insufficient to maintain either cell size or viability[J]. Mol Cell, 2000, 6(3): 683-692.
doi: 10.1016/s1097-2765(00)00066-6 pmid: 11030347 |
| [37] | KRAUSS S, BRAND M D, BUTTGEREIT F. Signaling takes a breath: new quantitative perspectives on bioenergetics and signal transduction[J]. Immunity, 2001, 15(4): 497-502. |
| [38] |
JACKSON A L, MATSUMOTO H, JANSZEN M, et al. Restricted expression of p55 interleukin 2 receptor (CD25) on normal T cells[J]. Clin Immunol Immunopathol, 1990, 54(1): 126-133.
pmid: 2403487 |
| [1] | TIAN Gaohui, ZHANG Qinxing, SHI Jiangzhou, ZHAO Fenfang, WANG Ning, ZHAO Jiaxuan, LU Yulin, XU Yao. A study on optimized lentiviral transduction conditions in CAR-T cells targeting CD30 [J]. China Oncology, 2023, 33(7): 646-654. |
| [2] | XUE Ying, MAO Yunyu, XU Jianqing. Progress in construction of hypoxia-sensitive CAR-T cell for solid tumor therapy [J]. China Oncology, 2023, 33(1): 71-77. |
| [3] | LI Fan, ZHANG Qinxing, TONG Xiangwen, TIAN Gaohui, GU Lixing, XU Yao. A study on influence of different signal peptides on anti-tumor effect of chimeric antigen receptor (CAR) T cells [J]. China Oncology, 2022, 32(2): 142-151. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
沪ICP备12009617
Powered by Beijing Magtech Co. Ltd
