Welcome to China Oncology,
China Oncology ›› 2022, Vol. 32 ›› Issue (7): 650-656.doi: 10.19401/j.cnki.1007-3639.2022.07.009
• Case Report • Previous Articles Next Articles
LIU Yanquan1,2( )(
)( ), HU Xiaomei3, YIN Yue4, LIN Lin5, SHEN Jianzhen4, CHEN Yuting1,2, TANG Huanwen1,2(
), HU Xiaomei3, YIN Yue4, LIN Lin5, SHEN Jianzhen4, CHEN Yuting1,2, TANG Huanwen1,2( )(
)( )
)
Received:2022-03-26
Online:2022-07-30
Published:2022-08-09
Contact:
TANG Huanwen
E-mail:doctorliuyanquan@163.com;thw@gdmu.edu.cn
Share article
CLC Number:
LIU Yanquan, HU Xiaomei, YIN Yue, LIN Lin, SHEN Jianzhen, CHEN Yuting, TANG Huanwen. A retrospective study and clinical analysis of post-transplant lymphoproliferative disorder[J]. China Oncology, 2022, 32(7): 650-656.
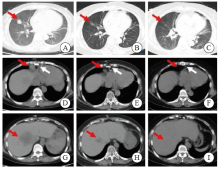
Fig. 1
CT images of chest and abdomen before and after treatment A: The red arrow indicated the space-occupying mass lesion; B, C: The red arrow indicated the disappearance of the space-occupying lesion after treatment; D: The red arrow indicated the enlarged lymph nodes in the heart and phrenic angle, and the white arrow indicated the postoperative imaging lesions of heart transplantation; E, F: The red arrow indicated the disappearance of enlarged lymph nodes, and the white arrow indicated postoperative changes of the sternum after heart transplantation; G: The red arrow indicated the liver mass and low-density lesions at the time of initial diagnosis (MT possible); H, I: the red arrow indicated the liver low-density lesions gradually decreased after treatment and disappear."
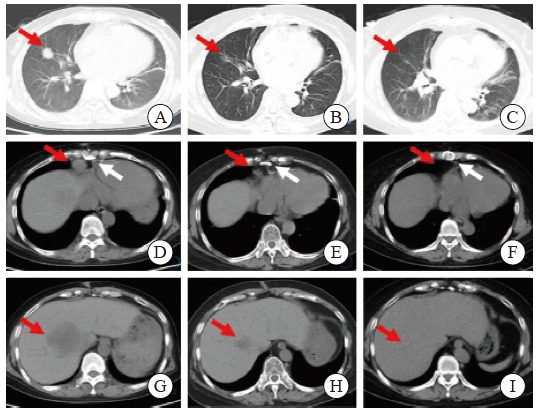
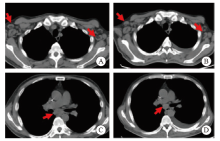
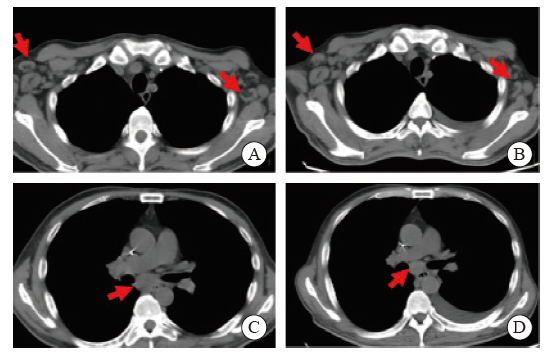
Fig. 4
Lung CT image of the patient A, C: Multiple enlarged lymph nodes in the mediastinum, bilateral supraclavicular area and bilateral axilla; B, D: The CT scans of the patient was automatically discharged from the hospital, it showed multiple enlarged lymph nodes in the mediastinum, bilateral supraclavicular area and bilateral axilla, similar to the previous."
| [1] | LAU E, MOYERS J T, WANG B C, et al. Analysis of post-transplant lymphoproliferative disorder (PTLD) outcomes with Epstein-Barr virus (EBV) assessments-a single tertiary referral center experience and review of literature[J]. Cancers (Basel), 2021, 13(4): 899. |
| [2] |
SWERDLOW S H, CAMPO E, PILERI S A, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms[J]. Blood, 2016, 127(20): 2375-2390.
doi: 10.1182/blood-2016-01-643569 |
| [3] |
STYCZYNSKI J, VAN DER VELDEN W, FOX C P, et al. Management of Epstein-Barr Virus infections and post-transplant lymphoproliferative disorders in patients after allogeneic hematopoietic stem cell transplantation: sixth European Conference on Infections in Leukemia (ECIL-6) guidelines[J]. Haematologica, 2016, 101(7): 803-811.
doi: 10.3324/haematol.2016.144428 |
| [4] |
SCHIEFER A I, SALZER E, FÜREDER A, et al. PD-L1 and PD1 expression in post-transplantation lymphoproliferative disease (PTLD) of childhood and adolescence: an inter- and intra-individual descriptive study covering the whole spectrum of PTLD categories[J]. Cancer Med, 2019, 8(10): 4656-4668.
doi: 10.1002/cam4.2394 |
| [5] |
PETRARA M R, GIUNCO S, SERRAINO D, et al. Post-transplant lymphoproliferative disorders: from epidemiology to pathogenesis-driven treatment[J]. Cancer Lett, 2015, 369(1): 37-44.
doi: 10.1016/j.canlet.2015.08.007 |
| [6] |
OPELZ G, HENDERSON R. Incidence of non-Hodgkin lymphoma in kidney and heart transplant recipients[J]. Lancet, 1993, 342(8886/8887): 1514-1516.
doi: 10.1016/S0140-6736(05)80084-4 |
| [7] |
COCKFIELD S M. Identifying the patient at risk for post-transplant lymphoproliferative disorder[J]. Transpl Infect Dis, 2001, 3(2): 70-78.
doi: 10.1034/j.1399-3062.2001.003002070.x |
| [8] |
OPELZ G, DÖHLER B. Lymphomas after solid organ transplantation: a collaborative transplant study report[J]. Am J Transplant, 2004, 4(2): 222-230.
doi: 10.1046/j.1600-6143.2003.00325.x |
| [9] |
WISTINGHAUSEN B, GROSS T G, BOLLARD C. Post-transplant lymphoproliferative disease in pediatric solid organ transplant recipients[J]. Pediatr Hematol Oncol, 2013, 30(6): 520-531.
doi: 10.3109/08880018.2013.798844 |
| [10] |
UHLIN M, WIKELL H, SUNDIN M, et al. Risk factors for Epstein-Barr virus-related post-transplant lymphoproliferative disease after allogeneic hematopoietic stem cell transplantation[J]. Haematologica, 2014, 99(2): 346-352.
doi: 10.3324/haematol.2013.087338 |
| [11] |
NAKANISHI C, KAWAGISHI N, SEKIGUCHI S, et al. Post-transplantation lymphoproliferative disorder in living-donor liver transplantation: a single-center experience[J]. Surg Today, 2012, 42(8): 741-751.
doi: 10.1007/s00595-012-0127-7 |
| [12] |
STYCZYNSKI J, GIL L, TRIDELLO G, et al. Response to rituximab-based therapy and risk factor analysis in Epstein Barr virus-related lymphoproliferative disorder after hematopoietic stem cell transplant in children and adults: a study from the infectious diseases working party of the European group for blood and marrow transplantation[J]. Clin Infect Dis, 2013, 57(6): 794-802.
doi: 10.1093/cid/cit391 |
| [13] |
DIERICKX D, HABERMANN T M. Post-transplantation lymphoproliferative disorders in adults[J]. N Engl J Med, 2018, 378(6): 549-562.
doi: 10.1056/NEJMra1702693 |
| [14] |
LUSKIN M R, HEIL D S, TAN K S, et al. The impact of EBV status on characteristics and outcomes of posttransplantation lymphoproliferative disorder[J]. Am J Transplant, 2015, 15(10): 2665-2673.
doi: 10.1111/ajt.13324 |
| [15] |
DESTEFANO C B, DESAI S H, SHENOY A G, et al. Management of post-transplant lymphoproliferative disorders[J]. Br J Haematol, 2018, 182(3): 330-343.
doi: 10.1111/bjh.15263 |
| [16] | MALONEY E M, BUSQUE V A, HUI S T, et al. Genomic variations in EBNA3C of EBV associate with posttransplant lymphoproliferative disorder[J]. JCI Insight, 2020, 5(6): 131644. |
| [17] |
GULLEY M L, TANG W H. Using Epstein-Barr viral load assays to diagnose, monitor, and prevent posttransplant lymphoproliferative disorder[J]. Clin Microbiol Rev, 2010, 23(2): 350-366.
doi: 10.1128/CMR.00006-09 |
| [18] | 中华医学会器官移植学分会. 器官移植受者EB病毒感染和移植后淋巴组织增生性疾病临床诊疗规范(2019版)[J]. 器官移植, 2019, 10(2): 149-157. |
| Branch of Organ Transplantation of Chinese Medical Association. Diagnosis and treatment specification for EB virus infection and posttransplant lymphoproliferative disease on recipients with organ transplantation in China (2019 edition)[J]. Organ Transplant, 2019, 10(2): 149-157. | |
| [19] |
KANAKRY J A, HEGDE A M, DURAND C M, et al. The clinical significance of EBV DNA in the plasma and peripheral blood mononuclear cells of patients with or without EBV diseases[J]. Blood, 2016, 127(16): 2007-2017.
doi: 10.1182/blood-2015-09-672030 |
| [20] |
KIMURA H, KWONG Y L. EBV viral loads in diagnosis, monitoring, and response assessment[J]. Front Oncol, 2019, 9: 62.
doi: 10.3389/fonc.2019.00062 |
| [21] |
ALLEN U D, PREIKSAITIS J K, AST INFECTIOUS DISEASES COMMUNITY OF PRACTICE. Epstein-Barr virus and posttransplant lymphoproliferative disorder in solid organ transplantation[J]. Am J Transplant, 2013, 13(Suppl 4): 107-120.
doi: 10.1111/ajt.12104 |
| [22] |
CAMPO E, SWERDLOW S H, HARRIS N L, et al. The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications[J]. Blood, 2011, 117(19): 5019-5032.
doi: 10.1182/blood-2011-01-293050 |
| [23] |
RAUSCH L, KOENECKE C, KOCH H F, et al. Matched-pair analysis: identification of factors with independent influence on the development of PTLD after kidney or liver transplantation[J]. Transplant Res, 2016, 5: 6.
doi: 10.1186/s13737-016-0036-1 |
| [24] | 刘彦权, 沈建箴, 刘庭波, 等. 第504例孕32周、贫血—顽固性溶血性贫血—重症肺部感染—心功能不全—左肾占位—弥漫大B细胞淋巴瘤[J]. 中华医学杂志, 2020, 100(20): 1588-1592. |
| LIU Y Q, SHEN J Z, LIU T B, et al. Case 504, 32 weeks of gestation, anemia-refractory hemolytic anemia-severe pulmonary infection-cardiac dysfunction-left renal mass-diffuse large B-cell lymphoma[J]. Natl Med J China, 2020, 100(20): 1588-1592. | |
| [25] | READY E, CHERNUSHKIN K, PARTOVI N, et al. Posttransplant lymphoproliferative disorder in adults receiving kidney transplantation in British Columbia: a retrospective cohort analysis[J]. Can J Kidney Health Dis, 2018, 5: 2054358118760831. |
| [26] |
TAOKA K, NANNYA Y, YAMAMOTO G, et al. Progressive transition of Epstein-Barr virus associated lymphoproliferative disease subtypes with the development of lung cancer[J]. Am J Hematol, 2009, 84(9): 600-603.
doi: 10.1002/ajh.21479 |
| [27] | AFSHAR K, RAO A P, PATEL V, et al. Use of foscarnet therapy for EBV infection following control of PTLD with enhancement of cellular immunity in a lung-transplant recipient[J]. J Transplant, 2011, 2011: 919651. |
| [28] |
HÖCKER B, BÖHM S, FICKENSCHER H, et al. (Val-) Ganciclovir prophylaxis reduces Epstein-Barr virus primary infection in pediatric renal transplantation[J]. Transpl Int, 2012, 25(7): 723-731.
doi: 10.1111/j.1432-2277.2012.01485.x |
| [29] |
PETERS A C, AKINWUMI M S, CERVERA C, et al. The changing epidemiology of posttransplant lymphoproliferative disorder in adult solid organ transplant recipients over 30 years: a single-center experience[J]. Transplantation, 2018, 102(9): 1553-1562.
doi: 10.1097/TP.0000000000002146 |
| [30] |
PARKER A, BOWLES K, BRADLEY J A, et al. Management of post-transplant lymphoproliferative disorder in adult solid organ transplant recipients-BCSH and BTS guidelines[J]. Br J Haematol, 2010, 149(5): 693-705.
doi: 10.1111/j.1365-2141.2010.08160.x |
| [31] |
TSAI D E, HARDY C L, TOMASZEWSKI J E, et al. Reduction in immunosuppression as initial therapy for posttransplant lymphoproliferative disorder: analysis of prognostic variables and long-term follow-up of 42 adult patients[J]. Transplantation, 2001, 71(8): 1076-1088.
doi: 10.1097/00007890-200104270-00012 |
| [32] |
GHOBRIAL I M, HABERMANN T M, RISTOW K M, et al. Prognostic factors in patients with post-transplant lymphoproliferative disorders (PTLD) in the rituximab era[J]. Leuk Lymphoma, 2005, 46(2): 191-196.
doi: 10.1080/10428190400012011 |
| [33] |
CHOQUET S, LEBLOND V, HERBRECHT R, et al. Efficacy and safety of rituximab in B-cell post-transplantation lymphoproliferative disorders: results of a prospective multicenter phase 2 study[J]. Blood, 2006, 107(8): 3053-3057.
doi: 10.1182/blood-2005-01-0377 |
| [34] |
BISHNOI R, BAJWA R, FRANKE A J, et al. Post-transplant lymphoproliferative disorder (PTLD): single institutional experience of 141 patients[J]. Exp Hematol Oncol, 2017, 6: 26.
doi: 10.1186/s40164-017-0087-0 |
| [35] | TRAPPE R U, DIERICKX D, ZIMMERMANN H, et al. Response to rituximab induction is a predictive marker in B-cell post-transplant lymphoproliferative disorder and allows successful stratification into rituximab or R-CHOP consolidation in an international, prospective, multicenter phase Ⅱ trial[J]. J Clin Oncol, 2017, 35(5): 536-543. |
| [36] |
TRAPPE R, OERTEL S, LEBLOND V, et al. Sequential treatment with rituximab followed by CHOP chemotherapy in adult B-cell post-transplant lymphoproliferative disorder (PTLD): the prospective international multicentre phase 2 PTLD-1 trial[J]. Lancet Oncol, 2012, 13(2): 196-206.
doi: 10.1016/S1470-2045(11)70300-X |
| [37] | MYNAREK M, SCHOBER T, BEHRENDS U, et al. Posttransplant lymphoproliferative disease after pediatric solid organ transplantation[J]. Clin Dev Immunol, 2013, 2013: 814973. |
| [38] |
GROSS T G, ORJUELA M A, PERKINS S L, et al. Low-dose chemotherapy and rituximab for posttransplant lymphoproliferative disease (PTLD): a children’s oncology group report[J]. Am J Transplant, 2012, 12(11): 3069-3075.
doi: 10.1111/j.1600-6143.2012.04206.x |
| [1] | WU Wen, ZHANG Ruoxin, WENG Junyong, MA Yanlei, CAI Guoxiang, LI Xinxiang, YANG Yongzhi. Exploring the prognostic value of positive lymph node ratio in stage Ⅲ colorectal cancer patients and establishing a predictive model [J]. China Oncology, 2024, 34(9): 873-880. |
| [2] | XIAO Feng, XU Tonglin, ZHU Lin, XIAO Jingwen, WU Tianqi, GU Chunyan. Significance of infiltration of M1 tumor-associated macrophages in hepatocellular carcinoma [J]. China Oncology, 2024, 34(8): 726-733. |
| [3] | ZHANG Ruoxin, YE Zilan, WENG Junyong, LI Xinxiang. Correlation study between advanced age and inferior prognosis in stage Ⅱ colorectal cancer patients [J]. China Oncology, 2024, 34(5): 485-492. |
| [4] | MA Fenghua, JIANG Anqi, CHEN Yiqing, XU Congjian, KANG Yu. Magnetic resonance imaging for distinguishing gastric-type endocervical adenocarcinoma from lobular endocervical glandular hyperplasia [J]. China Oncology, 2024, 34(4): 380-388. |
| [5] | LI Jun, LU Tingwei, FANG Xuqian. Impact of MSI-H/dMMR on clinicopathological characteristics and prognosis of patients with BRAF V600E-mutated resectable colorectal cancer [J]. China Oncology, 2024, 34(11): 1061-1066. |
| [6] | JIN Yizi, LIN Mingxi, ZHANG Jian. Receptor discordance between primary breast cancer and liver metastases [J]. China Oncology, 2023, 33(9): 834-843. |
| [7] | WU Han, YANG Zhangru, FENG Wen, ZENG Wanqin, GUO Jindong, LI Hongxuan, WANG Changlu, WANG Jiaming, LÜ Changxing, ZHANG Qin, YU Wen, CAI Xuwei, FU Xiaolong. The efficacy and prognosis analysis after stereotactic body radiotherapy for multiple primary early-stage lung cancer [J]. China Oncology, 2023, 33(9): 844-856. |
| [8] | CHEN Jinjuan, WANG Xingran, LI Wenzhi, CHENG Yu, SUN Yihua, TAO Xiang, MA Fenghua, SUN Li, ZHAO Hongbo, LU Xin. Conservative surgery in stage I placental site trophoblastic tumor: a report of 10 cases and literature review [J]. China Oncology, 2023, 33(9): 857-865. |
| [9] | SUN Yang, WANG Lian, ZHAO Meng, ZHANG Xiaofeng, GENG Zhijun, WANG Yueyue, SONG Xue, ZUO Lugen, LI Jing, HU Jianguo. The prognostic value of high expression of FKBP1A in gastric cancer and the regulatory effect of targeted PI3K/AKT on glucose metabolism [J]. China Oncology, 2023, 33(8): 726-739. |
| [10] | JIANG Lin, LIU Qiying, JIA Liqing, ZHANG Jing, CHANG Heng, XUE Tian, REN Min, BAI Qianming, ZHU Xiaoli, ZHOU Xiaoyan. Retrospective study on MGMT methylation status and its clinical significance in gliomas [J]. China Oncology, 2023, 33(8): 740-750. |
| [11] | WANG Ruoxi, JI Peng, GONG Yue, CHEN Sheng. Response rate and clinical outcome of HER2-low breast cancer after neoadjuvant therapy: a single-center retrospective study [J]. China Oncology, 2023, 33(7): 686-692. |
| [12] | ZUO Xueliang, CHEN Zhiqiang, DONG Runyu, WANG Zhixiong, CAI Juan. The value of combined detection of LDHA and PD-L1 in predicting the efficacy and prognosis of advanced gastric cancer patients treated with PD-1 inhibitor [J]. China Oncology, 2023, 33(5): 460-468. |
| [13] | CHEN Yuguang, SUN Xiao, BI Zhao, QIU Pengfei, DUAN Baowei, FAN Qingda, WANG Yongsheng. Internal mammary sentinel lymph node biopsy for breast cancer: a long-term follow-up research for assessment of prognosis and guiding individualized internal mammary lymph node irradiation [J]. China Oncology, 2023, 33(2): 142-151. |
| [14] | CHEN Yingyao, CHU Xiangling, YU Xin, SU Chunxia. Advances in models predicting efficacy of immune checkpoint inhibitors [J]. China Oncology, 2023, 33(1): 61-70. |
| [15] | GAO Heli, XU Jin, YU Xianjun. Updates on the research and management of pancreatic neuroendocrine neoplasm in 2021 [J]. China Oncology, 2022, 32(9): 772-778. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
沪ICP备12009617
Powered by Beijing Magtech Co. Ltd
