Welcome to China Oncology,
China Oncology ›› 2023, Vol. 33 ›› Issue (3): 250-259.doi: 10.19401/j.cnki.1007-3639.2023.03.008
• Article • Previous Articles Next Articles
ZHAO Manying1( ), WU Dongyue2, DU Ruiting2, YIN Lu3, LUO Yulu1(
), WU Dongyue2, DU Ruiting2, YIN Lu3, LUO Yulu1( )
)
Received:2022-05-11
Revised:2022-08-06
Online:2023-03-30
Published:2023-04-17
Contact:
LUO Yulu
Share article
CLC Number:
ZHAO Manying, WU Dongyue, DU Ruiting, YIN Lu, LUO Yulu. Mechanism of METTL14-mediated ERα m6A regulation of endometrial cancer metastasis[J]. China Oncology, 2023, 33(3): 250-259.

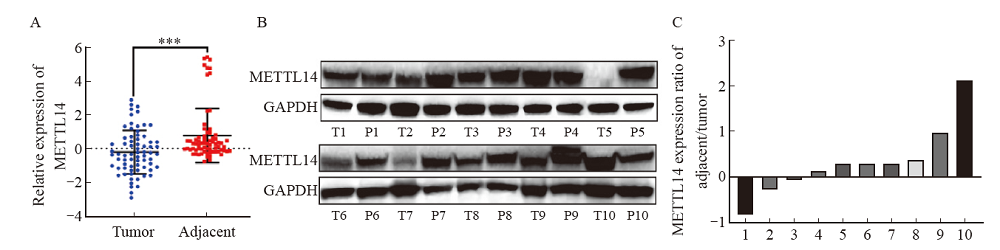
Fig. 1
mRNA and protein levels of METTL14 in EC were significantly down-regulated A: METTL14 mRNA expression was measured in tumor and normal tissues based on 70 pairs of EC samples. B, C: Representative plots and grayscale analysis of western blot analysis of METTL14 (calculated by the log2 ratio of EC and matched adjacent tissue, normalized to GAPDH) of 10 pairs of EC samples. ***: P<0.001. T: Tumor tissue; P: Paracancerous tissue."
Tab. 1
Association between METTL14 expression and clinicopathologic parameters"
| Clinicopathologic parameter | Number of patients n | Expression of METTL14 n | χ2 value | P value | |
|---|---|---|---|---|---|
| High | Low | ||||
| Patients | 96 | 40 | 56 | ||
| Age/year | 0.841 | 0.593 | |||
| <60 | 38 | 18 | 20 | ||
| ≥60 | 58 | 22 | 36 | ||
| Histology type | - | 0.192 | |||
| Grade 1 endometrioid | 38 | 20 | 18 | ||
| Grade 2 endometrioid | 27 | 9 | 18 | ||
| Grade 3 endometrioid | 23 | 8 | 15 | ||
| UPSC | 8 | 3 | 5 | ||
| FIGO stage | - | <0.001 | |||
| Ⅰ-Ⅱ | 68 | 35 | 33 | ||
| Ⅲ-Ⅳ | 28 | 5 | 23 | ||
| Depth of invasion | 6.796 | 0.018 | |||
| <50% | 42 | 25 | 17 | ||
| ≥50% | 54 | 15 | 39 | ||
| lymphovascular invasion | 8.820 | <0.001 | |||
| Negative | 50 | 28 | 22 | ||
| Positive | 46 | 12 | 34 | ||
| Lymph node metastasis | - | <0.001 | |||
| Negative | 74 | 38 | 36 | ||
| Positive | 22 | 2 | 20 | ||
| Metastasis tumor | - | <0.001 | |||
| Uterine tumor without metastasis | 66 | 32 | 34 | ||
| Uterine tumor with metastasis | 30 | 5 | 25 | ||
| Abdominal metastasis | 24 | 1 | 23 | ||
| Lymph node metastasis | 22 | 2 | 20 | ||
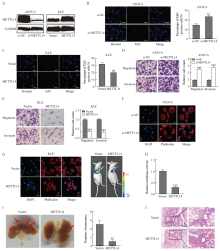
Fig. 2
METTL14 inhibited EC invasion and metastasis A: Western blot validation of METTL14 silencing and overexpression in AN3CA and KLE cells. B, C: EdU assay was used to determine the effects of METTL14 silencing and overexpression on the proliferation of AN3CA and KLE cells, respectively. D, E: Transwell analysis of the effects of METTL14 silencing and overexpression on the migration and invasion of AN3CA and KLE cells, respectively. F, G: Immunofluorescence imaging detected the cytoskeletal changes of AN3CA and KLE by METTL14 silencing and overexpression, respectively. Phalloidin (red) was used for cytoskeleton staining and DAPI (blue) was used to label nuclei (scale bar=30 μm). H: Representative in vivo images of mice were taken by quantifying luciferase activity in lung regions. I: Metastatic tumor foci in the lungs were photographed and quantified. J: The presence of metastatic tumor lesions was confirmed by H-E staining (scale bar=100 μm). **: P<0.01, compared with Vector group or si-NC group; ***: P<0.001, compared with Vector group or si-NC group."
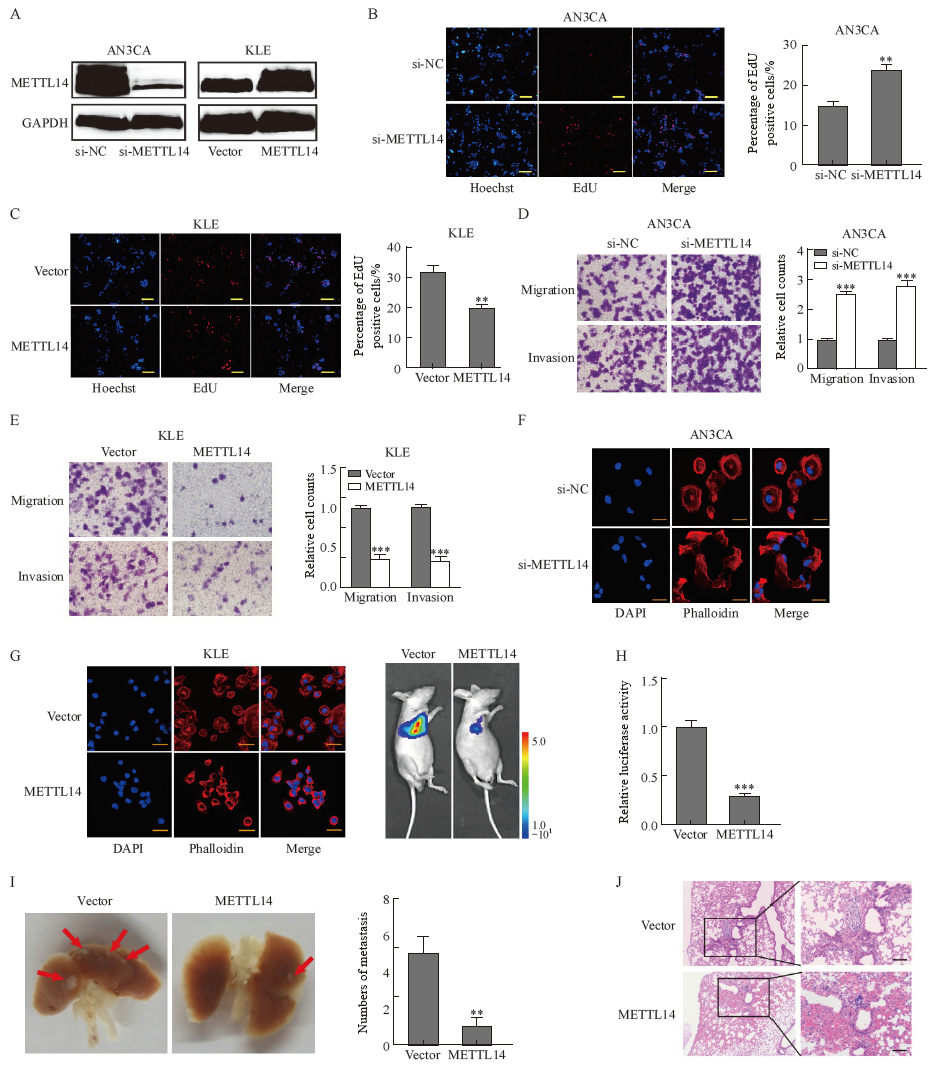
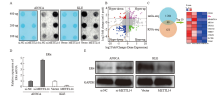
Fig. 3
ERα identified as a candidate target for METTL14 A: Overall m6A levels of RNA extracted from METTL14 knockdown or overexpressing EC cells were measured by m6A dot blot analysis. Methylene blue staining (left) was used to detect input RNA, and the intensity of dot immunoblotting (right) represents the level of m6A modification. B: Star chart showing the distribution of genes with differential (high or low) m6A peaks (Y axis; fold change >1.5 or <2/3, P<0.05) and differential (up or down) expression (X axis; fold change). Blue dots highlighted with circles represent down-regulated transcripts with reduced m6A abundance upon METTL14 overexpression, which were selected for the following studies. C: The results of MeRIP-seq (blue circles) and RNA-seq (brown circles) were combined using a Venn diagram. The overlap contains 60 transcripts affected by METTL14 for m6A content and expression. The top 10 differentially expressed genes shown in the heatmap (red for up-regulation, blue for down-regulation). D: RNA levels of ERα were detected in METTL14 silenced or overexpressed cells using RTFQ-PCR. E: The effects of METTL14 silencing and overexpression on the protein levels of ERα in AN3CA and KLE cells were detected by Western blot, respectively."

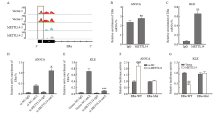
Fig. 4
METTL14 destabilized ERα by regulating m6A modification A: IGV plotted m6A abundance on ERα mRNA in negative control or METTL14-overexpressing KLE cells. Green and pink represent the m6A signal of the input sample, while red and blue represent the signal of the IP sample. Black blocks indicate sites where m6A levels differ between the two groups, with the most significant locations highlighted with grey panes. B, C: Relative enrichment of ERα mRNA associated with METTL14 protein was identified by RIP assay using anti-IgG and anti-METTL14 antibodies. The IgG group was a negative control to exclude nonspecific binding. The Y-axis represents the percentage of input for each IP sample. **: P<0.01, compared with the IgG group. D, E: Detection of m6A modification of ERα by MeRIP-RTFQ-PCR analysis using anti-IgG and anti-m6A antibodies. *: P<0.05, compared with si-NC-m6A group or Vector-m6A group; ***: P <0.001, compared with si-NC-m6A group or Vector-m6A group. F, G: Relative luciferase activity of AN3CA and KLE cells transfected with ERα-wild-type or mutant constructs. ##: P<0.01, compared with Vector group or si-NC group; ###: P<0.001, compared with Vector group or si-NC group."
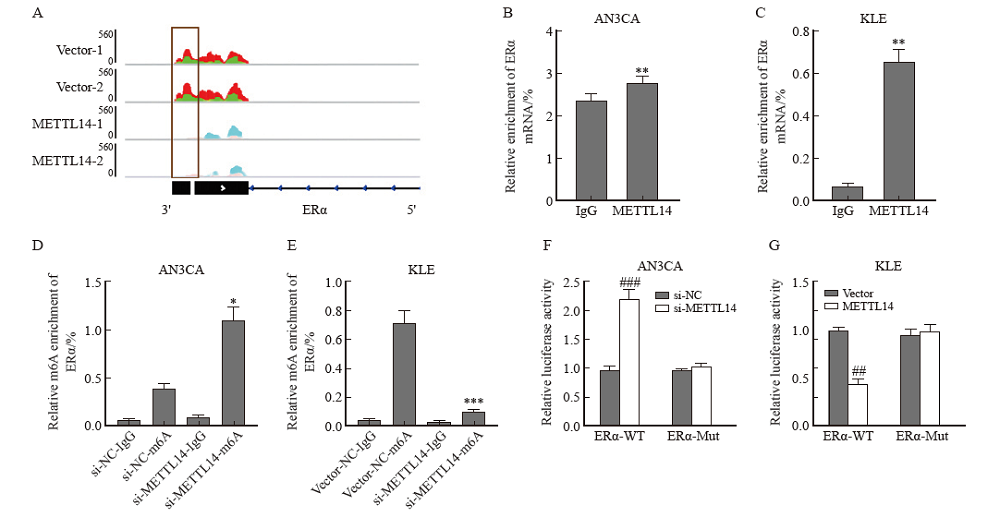

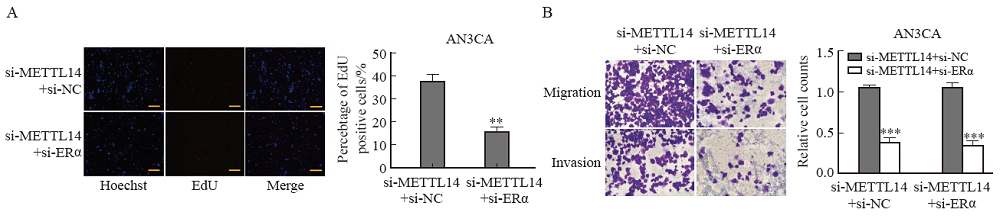
Fig. 5
ERα knockdown reversed the effect of METTL14 on EC cell proliferation or migration A: EdU assayed the effect of METTL14 silencing and ERα silencing on the proliferation of AN3CA cells. B: Transwell analysis of the effects of METTL14 silencing and ERα silencing on the migration and invasion of AN3CA cells. **: P<0.01, compared with si-METTL14+si-NC group; ***: P <0.001, compared with si-METTL14+si-NC group."
| [1] |
URICK M E, BELL D W. Clinical actionability of molecular targets in endometrial cancer[J]. Nat Rev Cancer, 2019, 19(9): 510-521.
doi: 10.1038/s41568-019-0177-x pmid: 31388127 |
| [2] |
DE BOER S M, POWELL M E, MILESHKIN L, et al. Adjuvant chemoradiotherapy versus radiotherapy alone in women with high-risk endometrial cancer (PORTEC-3): patterns of recurrence and post-hoc survival analysis of a randomised phase 3 trial[J]. Lancet Oncol, 2019, 20(9): 1273-1285.
doi: S1470-2045(19)30395-X pmid: 31345626 |
| [3] | LI M, ZHA X, WANG S J. The role of N6-methyladenosine mRNA in the tumor microenvironment[J]. Biochim Biophys Acta Rev Cancer, 2021, 1875(2): 188522. |
| [4] |
LI J M, LIANG L, YANG Y Z, et al. N6-methyladenosine as a biological and clinical determinant in colorectal cancer: progression and future direction[J]. Theranostics, 2021, 11(6): 2581-2593.
doi: 10.7150/thno.52366 |
| [5] |
YANG X, ZHANG S, HE C Y, et al. METTL14 suppresses proliferation and metastasis of colorectal cancer by down-regulating oncogenic long non-coding RNA XIST[J]. Mol Cancer, 2020, 19(1): 46.
doi: 10.1186/s12943-020-1146-4 pmid: 32111213 |
| [6] |
SHEN S, YAN J Y, ZHANG Y Z, et al. N6-methyladenosine (m6A)-mediated messenger RNA signatures and the tumor immune microenvironment can predict the prognosis of hepatocellular carcinoma[J]. Ann Transl Med, 2021, 9(1): 59.
doi: 10.21037/atm-20-7396 pmid: 33553352 |
| [7] | 韩娟娟, 张新安, 艾福录. m6A RNA甲基化修饰异常在肿瘤中的作用[J]. 中国生物化学与分子生物学报, 2020, 36(4): 383-391. |
| HAN J J, ZHANG X N, AI F L. The role of m6A RNA methylated abnormality in tumor[J]. Chin J Biochem Mol Biol, 2020, 36(4): 383-391. | |
| [8] |
MA J Z, YANG F, ZHOU C C, et al. METTL14 suppresses the metastatic potential of hepatocellular carcinoma by modulating N6-methyladenosine-dependent primary microRNA processing[J]. Hepatology, 2017, 65(2): 529-543.
doi: 10.1002/hep.28885 |
| [9] |
LI Q, WANG C Y, DONG W, et al. WTAP facilitates progression of endometrial cancer via CAV-1/NF-κB axis[J]. Cell Biol Int, 2021, 45(6): 1269-1277.
doi: 10.1002/cbin.11570 pmid: 33559954 |
| [10] |
MA J, YANG D, MA X X. Immune infiltration-related N6-methyladenosine RNA methylation regulators influence the malignancy and prognosis of endometrial cancer[J]. Aging, 2021, 13(12): 16287-16315.
doi: 10.18632/aging.v13i12 |
| [11] |
WENG H Y, HUANG H L, WU H Z, et al. METTL14 inhibits hematopoietic stem/progenitor differentiation and promotes leukemogenesis via mRNA m6A modification[J]. Cell Stem Cell, 2018, 22(2): 191-205.e9.
doi: 10.1016/j.stem.2017.11.016 |
| [12] |
WEI J C, HARADA B T, LU D, et al. HRD1-mediated METTL14 degradation regulates m6A mRNA modification to suppress ER proteotoxic liver disease[J]. Mol Cell, 2021, 81(24): 5052-5065.e6.
doi: 10.1016/j.molcel.2021.10.028 |
| [13] | QIN F, CAI B S, ZHAO J, et al. Methyltransferase-like protein 14 attenuates mitochondrial antiviral signaling protein expression to negatively regulate antiviral immunity via N6-methyladenosine modification[J]. Adv Sci (Weinh), 2021, 8(15): e2100606. |
| [14] | PANG P, QU Z Z, YU S T, et al. Mettl14 attenuates cardiac ischemia/reperfusion injury by regulating Wnt1/β-catenin signaling pathway[J]. Front Cell Dev Biol, 2021, 9: 762853. |
| [15] | GALARDI S, MICHIENZI A, CIAFRÈ S A. Insights into the regulatory role of m6A epitranscriptome in glioblastoma[J]. Int J Mol Sci, 2020, 21(8): E2816. |
| [16] |
SUN T, WU Z K, WANG X F, et al. LNC942 promoting METTL14-mediated m6A methylation in breast cancer cell proliferation and progression[J]. Oncogene, 2020, 39(31): 5358-5372.
doi: 10.1038/s41388-020-1338-9 |
| [17] | YAO Q, HE L Z, GAO X C, et al. The m6A methyltransferase METTL14-mediated N6-methyladenosine modification of PTEN mRNA inhibits tumor growth and metastasis in stomach adenocarcinoma[J]. Front Oncol, 2021, 11: 699749. |
| [18] |
CHENG Y, WANG M Q, ZHOU J L, et al. The important role of N6-methyladenosine RNA modification in non-small cell lung cancer[J]. Genes (Basel), 2021, 12(3): 440.
doi: 10.3390/genes12030440 |
| [19] |
KASOHA M, DERNEKTSI C, SEIBOLD A, et al. Crosstalk of estrogen receptors and Wnt/β-catenin signaling in endometrial cancer[J]. J Cancer Res Clin Oncol, 2020, 146(2): 315-327.
doi: 10.1007/s00432-019-03114-8 pmid: 31865530 |
| [20] |
JING X X, PENG J, DOU Y, et al. Macrophage ERα promoted invasion of endometrial cancer cell by mTOR/KIF5B-mediated epithelial to mesenchymal transition[J]. Immunol Cell Biol, 2019, 97(6): 563-576.
doi: 10.1111/imcb.12245 pmid: 30779215 |
| [21] |
CHEN Z, YANG H J, LIN Q, et al. Estrogen-ERα signaling and DNA hypomethylation co-regulate expression of stem cell protein PIWIL1 in ERα-positive endometrial cancer cells[J]. Cell Commun Signal, 2020, 18(1): 84.
doi: 10.1186/s12964-020-00563-4 pmid: 32503542 |
| [1] | FENG Zheng, GUO Qinhao, ZHU Jun, WU Xiaohua, WEN Hao. Progress in treatment of gynecological cancer in 2023 [J]. China Oncology, 2024, 34(4): 340-360. |
| [2] | DIAO Xinfeng, LI Xinmao, HOU Liang, WEI Zhixuan. WTAP promotes proliferation and aerobic glycolysis via regulating m6A modification of BMI1 mRNA [J]. China Oncology, 2023, 33(7): 655-663. |
| [3] | XIA Lingfang, ZHU Jun, WU Xiaohua. The latest progress and prospect of gynecological tumor treatment at 2023 ESMO [J]. China Oncology, 2023, 33(11): 969-980. |
| [4] | GUO Qinhao, YU Min, WU Xiaohua. Progress in diagnosis and treatment of gynecological tumors in 2022 [J]. China Oncology, 2023, 33(1): 14-24. |
| [5] | ZHAO Mingming, WANG Tianyou, WANG Chao. Progress in menopausal hormone therapy for postoperative patients with gynecological malignant cancer [J]. China Oncology, 2022, 32(11): 1098-1104. |
| [6] | The Society of Gynecological Cancer of China Anti-Cancer Association, Chinese Society of Pathology of the Chinese Medical Association, National Pathology Quality Control Center. The Chinese Expert Consensus Recommendations on Molecular Testing in Endometrial Cancer (2021 edition) [J]. China Oncology, 2021, 31(11): 1126-1144. |
| [7] | LU Yuanyuan, YANG Fan, ZHENG Ying. Research progress and prospect of sentinel lymph node mapping in endometrial carcinoma [J]. China Oncology, 2021, 31(10): 944-948. |
| [8] | WEN Jing , HUANG Jie , LI Yunyun , ZHANG Zhongzu , ZHOU Qin . Effects of tumor-associated macrophage-related miR-99a on the cell growth and invasion of endometrial cancer cells [J]. China Oncology, 2020, 30(8): 561-569. |
| [9] | YANG Guang-dong,REN Yuan, ZHANG Rong. Identify effect of SERPINA3 up-regulated expression in endometrial cancer [J]. China Oncology, 2014, 24(4): 284-291. |
| [10] | LU Yuan-yuan,ZHANG Jie-qing,LIANG Shao-feng,LI Li. Estradiol enhances the proliferation of endometrial cancer cells by producing angiogenesis by activating AKT pathway [J]. China Oncology, 2013, 23(11): 868-873. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
沪ICP备12009617
Powered by Beijing Magtech Co. Ltd
