Welcome to China Oncology,
China Oncology ›› 2023, Vol. 33 ›› Issue (2): 152-161.doi: 10.19401/j.cnki.1007-3639.2023.02.008
• Article • Previous Articles Next Articles
LENG Jie( ), QIU Guochun, ZHANG Bo, PU Yan
), QIU Guochun, ZHANG Bo, PU Yan
Received:2022-08-03
Revised:2022-12-03
Online:2023-02-28
Published:2023-03-22
Contact:
LENG Jie
Share article
CLC Number:
LENG Jie, QIU Guochun, ZHANG Bo, PU Yan. Mechanism of breast cancer centrosome regulatory protein SEC23B on tumor invasion and metastasis[J]. China Oncology, 2023, 33(2): 152-161.

Fig. 1
SEC23B is upregulated in metastatic breast cancer A: Kaplan-Meier survival analysis of the correlation between SEC23B expression levels and overall survival of breast cancer patients was performed in an online database (n = 4 929); B: Association between SEC23B expression and breast cancer metastasis was analyzed using the HCMDB database box line (n = 368); C: SEC23B expression in breast cancer cells was detected by Western blot."
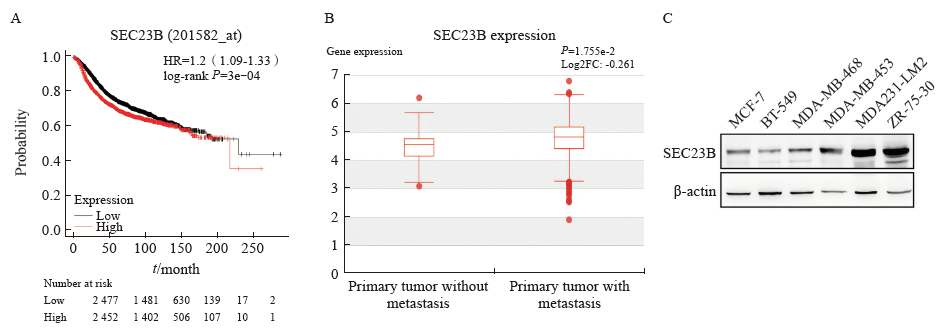
Fig. 2
SEC23B promotes breast cancer migration in vitro A: Transfection efficiency of SEC23B overexpression plasmid in MCF-7 cells was examined by Western blot; B-C: Effect of SEC23B overexpression on MCF-7 cell migration was assessed by transwell assay and wound healing assay; D: The expression of sh-SEC23B lentivirus in MDA231-LM2 cells by Western blot; E-F: Effect of SEC23B knockdown on migration of MDA231-LM2 cells was assessed by transwell assay and wound healing assay. ***: P<0.001, compared to control or sh-NC groups."
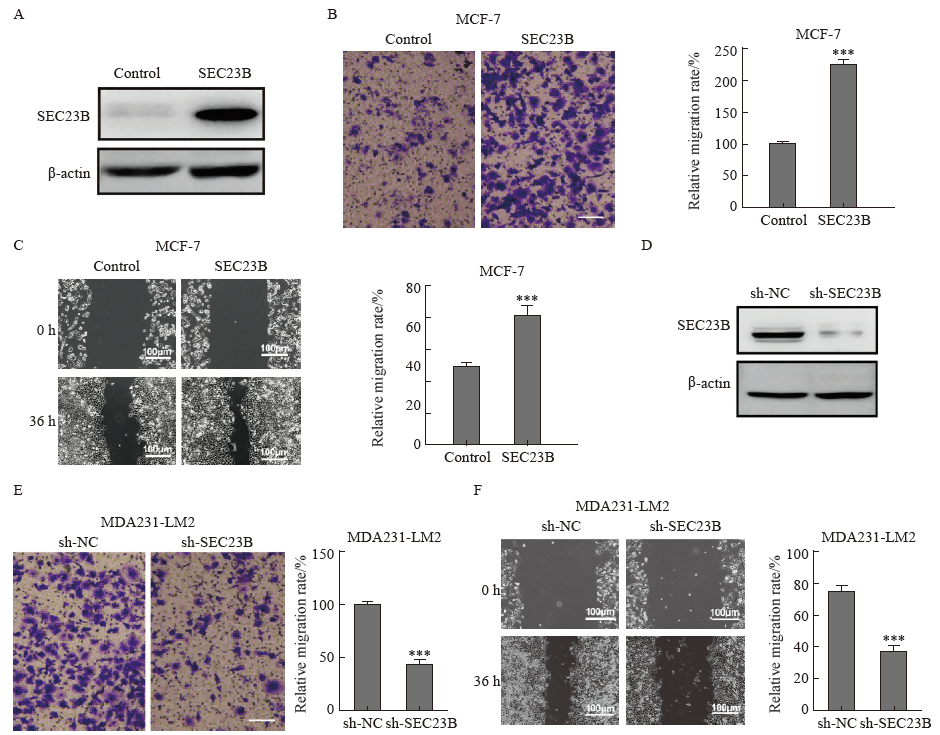
Fig. 3
SEC23B induces autophagy in breast cancer cells A: Effect of SEC23B overexpression plasmid on p62, LCA/B expression in MCF-7 cells detected by Western blot; B: Representative images and quantitative analysis of the effect of SEC23B overexpression on autophagic granules in MCF-7 cells detected by immunofluorescence staining; C: Effect of SEC23B knockdown on p62, LCA/B expression in MDA231-LM2 cells by Western blot; D: Representative images and quantitative analysis of the effect of SEC23B knockdown on autophagic granules in MDA231-LM2 detected by immunofluorescence staining; E: Effect of CQ on migration of SEC23B overexpressing MCF-7 cells was assessed by transwell assay. **: P<0.01, compared with control or sh-NC groups; ***: P<0.001, compared with control or sh-NC groups."
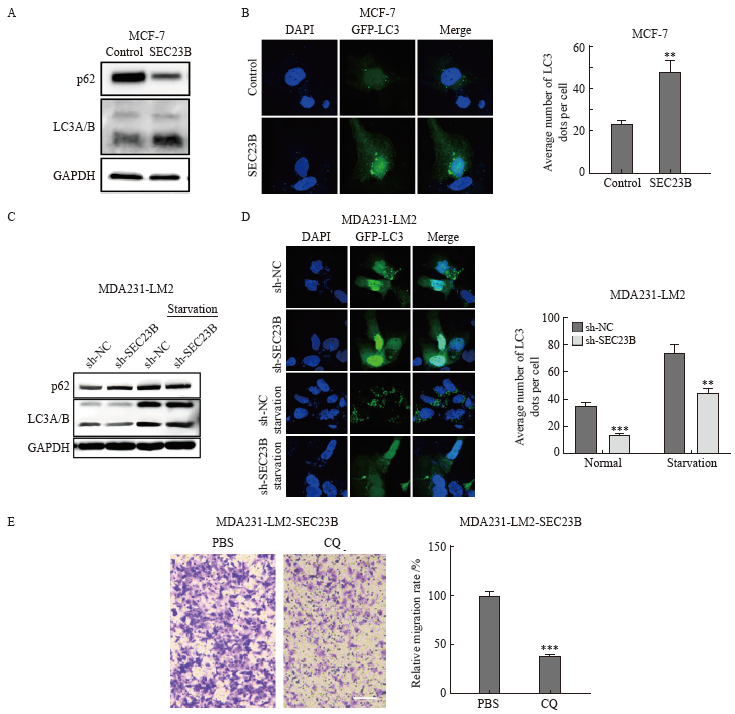
Fig. 4
ERK/mTOR pathway is involved in SEC23B-induced autophagy in breast cancer cells A: Effect of SEC23B overexpression plasmid on ERK/mTOR pathway expression in MCF-7 cells detected by Western blot; B: Effect of SEC23B knockdown on ERK/mTOR pathway expression in MDA231-LM2 cells detected by Western blot; C: Effect of EGF on ERK/mTOR pathway expression in MCF-7 cells detected by Western blot, effects of EGF on ERK/mTOR pathway and p62, LCA/B expression in SEC23B overexpressing MCF-7 cells detected by Western blot; D: Representative images and quantitative analysis of the effects of EGF on autophagic granules in SEC23B overexpressing MCF-7 cells detected by immunofluorescence staining. **: P<0.01, compared with the control group; #: P<0.05, compared with the SEC23B group."
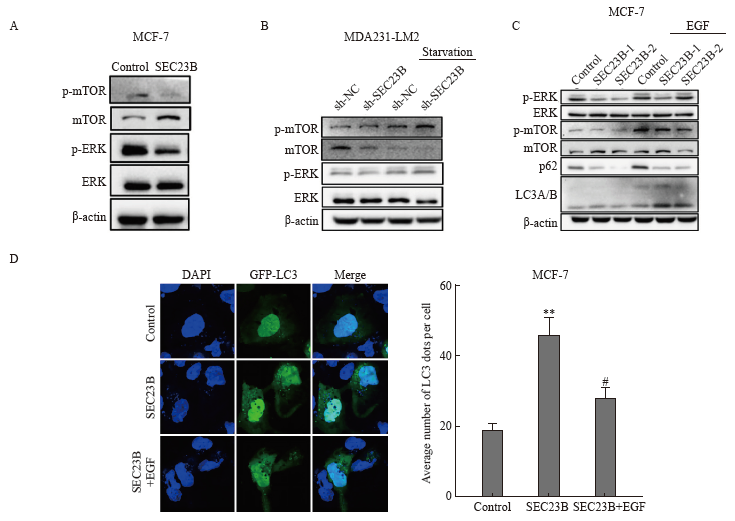
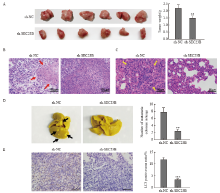
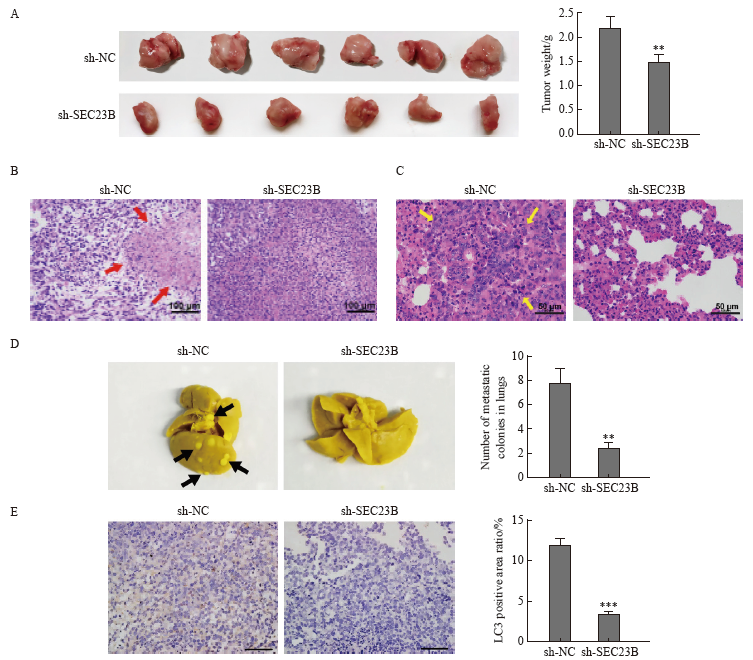
Fig. 5
SEC23B knockdown inhibits breast cancer metastasis and autophagy in vivo A: Tumors were excised 21 days after implantation and final tumor weight was recorded. B: Histological examination by H-E staining was performed to determine tumor morphology and structural changes. Tumor necrosis was indicated by a red arrow. C: Lung metastases were examined by H-E staining. Tumor metastases in lung tissue were indicated by yellow arrows. D: Lung tissue was excised and fixed in Bouin solution 21 days after implantation, and the number of metastatic nodules was recorded for each mouse. Metastatic nodules were indicated by black arrows. E: LC3 expression in tumor tissues was examined by H-E staining. **: P<0.01, compared with sh-NC group; ***: P<0.001, compared with sh-NC group."
| [1] | 王帅, 崔中豪, 杨毅. HER2阳性乳腺癌脑转移的靶向治疗研究进展[J]. 医学研究生学报, 2020, 33(2): 215-219. |
|
WANG S, CUI Z H, YANG Y. Research progress of targeted therapy for HER2 positive breast cancer with brain metastases[J]. J Med Postgrad, 2020, 33(2): 215-219.
doi: 10.1136/pgmj.33.379.215 |
|
| [2] | FARES J, FARES M Y, KHACHFE H H, et al. Molecular principles of metastasis: a hallmark of cancer revisited[J]. Signal Transduct Target Ther, 2020, 5(1): 28. |
| [3] |
ARAKEL E C, SCHWAPPACH B. Formation of COPI-coated vesicles at a glance[J]. J Cell Sci, 2018, 131(5): jcs209890.
doi: 10.1242/jcs.209890 |
| [4] |
WEI W, LIU Z G, ZHANG C, et al. A common human missense mutation of vesicle coat protein SEC23B leads to growth restriction and chronic pancreatitis in mice[J]. J Biol Chem, 2022, 298(1): 101536.
doi: 10.1016/j.jbc.2021.101536 |
| [5] |
ZHOU J G, SINGH P, YIN K H, et al. Non-medullary thyroid cancer susceptibility genes: evidence and disease spectrum[J]. Ann Surg Oncol, 2021, 28(11): 6590-6600.
doi: 10.1245/s10434-021-09745-x pmid: 33660127 |
| [6] |
RAMALHO-CARVALHO J, MARTINS J B, CEKAITE L, et al. Epigenetic disruption of miR-130a promotes prostate cancer by targeting SEC23B and DEPDC1[J]. Cancer Lett, 2017, 385: 150-159.
doi: 10.1016/j.canlet.2016.10.028 |
| [7] |
LIU Z, WANG Q, MAO J W, et al. Comparative proteomic analysis of protein methylation provides insight into the resistance of hepatocellular carcinoma to 5-fluorouracil[J]. J Proteomics, 2020, 219: 103738.
doi: 10.1016/j.jprot.2020.103738 |
| [8] | ZEYEN L, DÖRING T, STIELER J T, et al. Hepatitis B subviral envelope particles use the COPII machinery for intracellular transport via selective exploitation of Sec24A and Sec23B[J]. Cell Microbiol, 2020, 22(6): e13181. |
| [9] | 周慧, 韩翰, 周伟强. SAHA-CTSV轴通过诱导过度自噬抑制乳腺癌MCF-7细胞的生长[J]. 中国药理学通报, 2021, 37(4): 504-510. |
| ZHOU H, HAN H, ZHOU W Q. SAHA-CTSV induced excessive autophagy and inhibited growth of breast cancer MCF-7 cells[J]. Chin Pharmacol Bull, 2021, 37(4): 504-510. | |
| [10] |
MAITI A, HAIT N C. Autophagy-mediated tumor cell survival and progression of breast cancer metastasis to the brain[J]. J Cancer, 2021, 12(4): 954-964.
doi: 10.7150/jca.50137 pmid: 33442395 |
| [11] |
KING R, LIN Z S, BALBIN-CUESTA G, et al. SEC23A rescues SEC23B-deficient congenital dyserythropoietic anemia type Ⅱ[J]. Sci Adv, 2021, 7(48): eabj5293.
doi: 10.1126/sciadv.abj5293 |
| [12] |
YEHIA L, NIAZI F, NI Y, et al. Germline heterozygous variants in SEC23B are associated with cowden syndrome and enriched in apparently sporadic thyroid cancer[J]. Am J Hum Genet, 2015, 97(5): 661-676.
doi: 10.1016/j.ajhg.2015.10.001 pmid: 26522472 |
| [13] |
YEHIA L, JINDAL S, KOMAR A A, et al. Non-canonical role of cancer-associated mutant SEC23B in the ribosome biogenesis pathway[J]. Hum Mol Genet, 2018, 27(18): 3154-3164.
doi: 10.1093/hmg/ddy226 |
| [14] |
PIFFOUX M, ERIAU E, CASSIER P A. Autophagy as a therapeutic target in pancreatic cancer[J]. Br J Cancer, 2021, 124(2): 333-344.
doi: 10.1038/s41416-020-01039-5 |
| [15] |
DOWER C M, WILLS C A, FRISCH S M, et al. Mechanisms and context underlying the role of autophagy in cancer metastasis[J]. Autophagy, 2018, 14(7): 1110-1128.
doi: 10.1080/15548627.2018.1450020 pmid: 29863947 |
| [16] |
FAN Q, YANG L, ZHANG X D, et al. Autophagy promotes metastasis and glycolysis by upregulating MCT1 expression and Wnt/β-catenin signaling pathway activation in hepatocellular carcinoma cells[J]. J Exp Clin Cancer Res, 2018, 37(1): 9.
doi: 10.1186/s13046-018-0673-y |
| [17] |
JEONG Y T, SIMONESCHI D, KEEGAN S, et al. The ULK1-FBXW5-SEC23B nexus controls autophagy[J]. Elife, 2018, 7: e42253.
doi: 10.7554/eLife.42253 |
| [18] |
HUANG S F, TANG M Z, JIANG H L, et al. A COPII subunit interacting with ER-phagy receptor: a new potential avenue to maintaining neuronal homeostasis[J]. Acta Biochim Biophys Sin (Shanghai), 2020, 52(6): 698-700.
doi: 10.1093/abbs/gmaa031 |
| [19] |
XU H Y, LIU L Y, DING M, et al. Effect of Ganoderma applanatum polysaccharides on MAPK/ERK pathway affecting autophagy in breast cancer MCF-7 cells[J]. Int J Biol Macromol, 2020, 146: 353-362.
doi: S0141-8130(19)38698-2 pmid: 31911173 |
| [20] |
YANG C Y, CHEN N, LI X, et al. Mutations in the coat complex Ⅱ component SEC23B promote colorectal cancer metastasis[J]. Cell Death Dis, 2020, 11(3): 157.
doi: 10.1038/s41419-020-2358-7 |
| [1] | CHEN Yijun, LIU Yuhang, DUAN Haibo, WANG Xiongjun. Functional and mechanistic of AGPAT5 in liver cancer [J]. China Oncology, 2024, 34(9): 838-847. |
| [2] | XU Rui, WANG Zehao, WU Jiong. Advances in the role of tumor-associated neutrophils in the development of breast cancer [J]. China Oncology, 2024, 34(9): 881-889. |
| [3] | CAO Xiaoshan, YANG Beibei, CONG Binbin, LIU Hong. The progress of treatment for brain metastases of triple-negative breast cancer [J]. China Oncology, 2024, 34(8): 777-784. |
| [4] | ZHANG Jian. Clinical consideration of two key questions in assessing menopausal status of female breast cancer patients [J]. China Oncology, 2024, 34(7): 619-627. |
| [5] | JIANG Dan, SONG Guoqing, WANG Xiaodan. Study on the mechanism of mitochondrial dysfunction and CPT1A/ERK signal transduction pathway regulating malignant behavior in breast cancer [J]. China Oncology, 2024, 34(7): 650-658. |
| [6] | HUANG Sijie, KANG Xun, LI Wenbin. Clinical research progress of intrathecal therapy in the treatment of leptomeningeal metastasis [J]. China Oncology, 2024, 34(7): 695-701. |
| [7] | DONG Jianqiao, LI Kunyan, LI Jing, WANG Bin, WANG Yanhong, JIA Hongyan. A study on mechanism of SIRT3 inducing endocrine drug resistance in breast cancer via deacetylating YME1L1 [J]. China Oncology, 2024, 34(6): 537-547. |
| [8] | HAO Xian, HUANG Jianjun, YANG Wenxiu, LIU Jinting, ZHANG Junhong, LUO Yubei, LI Qing, WANG Dahong, GAO Yuwei, TAN Fuyun, BO Li, ZHENG Yu, WANG Rong, FENG Jianglong, LI Jing, ZHAO Chunhua, DOU Xiaowei. Establishment of primary breast cancer cell line as new model for drug screening and basic research [J]. China Oncology, 2024, 34(6): 561-570. |
| [9] | SHEN Jie, FENG Xiaoshuang, WEN Hao, ZHOU Changming, MO Miao, WANG Zezhou, YUAN Jing, WU Xiaohua, ZHENG Ying. Metastasis patterns and survival analysis of 572 patients with metastatic cervical cancer: a hospital-based real world study [J]. China Oncology, 2024, 34(4): 361-367. |
| [10] | LI Xiaohui, ZHAO Jiaxu, PENG Haibao, ZHANG Ye, ZENG Rui, CHI Yudan. Effects of HMGA2 on migration and proliferation of leptomeningeal metastatic melanoma [J]. China Oncology, 2024, 34(4): 389-399. |
| [11] | Committee of Breast Cancer Society, China Anti-Cancer Association. Expert consensus on clinical applications of ovarian function suppression for Chinese women with early breast cancer (2024 edition) [J]. China Oncology, 2024, 34(3): 316-333. |
| [12] | ZHANG Qi, XIU Bingqiu, WU Jiong. Progress of important clinical research of breast cancer in China in 2023 [J]. China Oncology, 2024, 34(2): 135-142. |
| [13] | ZHANG Siyuan, JIANG Zefei. Important research progress in clinical practice for advanced breast cancer in 2023 [J]. China Oncology, 2024, 34(2): 143-150. |
| [14] | WANG Zhaobu, LI Xing, YU Xinmiao, JIN Feng. Important research progress in clinical practice for early breast cancer in 2023 [J]. China Oncology, 2024, 34(2): 151-160. |
| [15] | LUO Yang, SUN Tao, SHAO Zhimin, CUI Jiuwei, PAN Yueyin, ZHANG Qingyuan, CHENG Ying, LI huiping, YANG Yan, YE Changsheng, YU Guohua, WANG Jingfen, LIU Yunjiang, LIU Xinlan, ZHOU Yuhong, BAI Yuju, GU Yuanting, WANG Xiaojia, XU Binghe, SONG Lihua. Efficacy, metabolic characteristics, safety and immunogenicity of AK-HER2 compared with reference trastuzumab in patients with metastatic HER2-positive breast cancer: a multicenter, randomized, double-blind phase Ⅲ equivalence trial [J]. China Oncology, 2024, 34(2): 161-175. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
沪ICP备12009617
Powered by Beijing Magtech Co. Ltd
