Welcome to China Oncology,
China Oncology ›› 2024, Vol. 34 ›› Issue (9): 881-889.doi: 10.19401/j.cnki.1007-3639.2024.09.008
• Review • Previous Articles Next Articles
XU Rui( ), WANG Zehao, WU Jiong(
), WANG Zehao, WU Jiong( )
)
Received:2024-05-20
Revised:2024-09-12
Online:2024-09-30
Published:2024-10-11
Contact:
WU Jiong
Share article
CLC Number:
XU Rui, WANG Zehao, WU Jiong. Advances in the role of tumor-associated neutrophils in the development of breast cancer[J]. China Oncology, 2024, 34(9): 881-889.
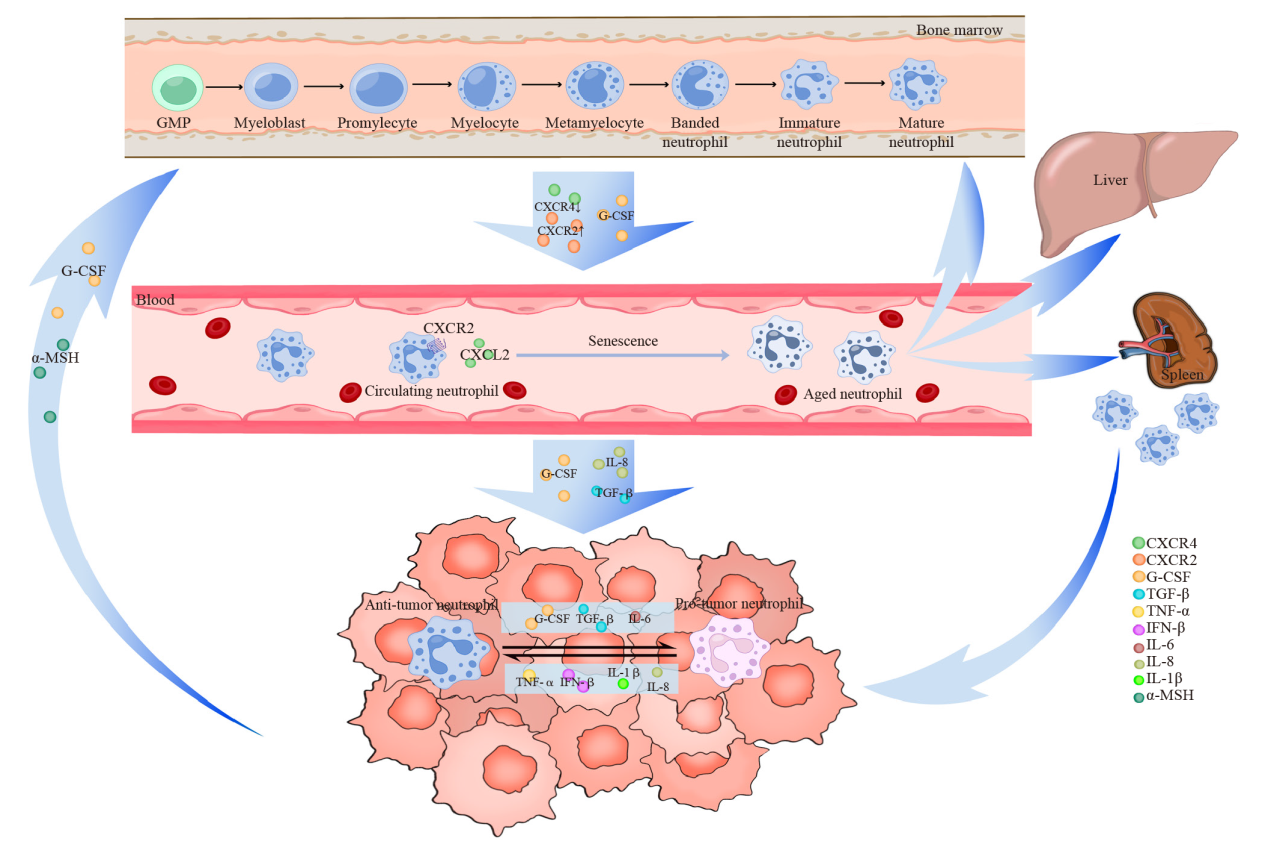
Fig. 1
The formation and function of TANs Neutrophils are derived from multipotent GMP located in the bone marrow, where they proliferate and mature into mature neutrophils before entering peripheral blood circulation. Within this circulation, neutrophils undergo aging processes to become aged neutrophils, which subsequently migrate back to the bone marrow, liver, and spleen for phagocytosis by macrophages. Various tumor-derived cytokines facilitate the recruitment of neutrophils into the TME, resulting in the formation of TANs. Additionally, tumor cells directly influence bone marrow activity to modulate TANs recruitment. TANs exhibit a dual role both promotes and inhibits tumor growth and dynamically convert between these two functional phenotypes through polarization. Numerous tumor-derived cytokines are involved in mediating this polarization effect."
Tab. 1
Phenotypic and functional differences of peripheral blood neutrophils and TANs in different tumors"
| Type | Phenotype | Function |
|---|---|---|
| NI | Human: CD66b+, CD11b+, CD117+, CD10-, CD16int/low, LOX1+, CD84+, JAML+; Mouse: Ly6G+, CD11b+, CD117+, CD170low, CD101-, CD84+, JAML+ | Tumor immunosuppression |
| N1 type | Human: CD66b+, CD11b+, CD101+ CD177+ (in CRC), CD54+, HLA-DR+, CD86+, CD15high; Mouse: CD11b+, CD177+ (in CRC), CD54+, CD16+, CD170low, Ly6G+ | Cytotoxicity; Inhibit tumor invasion |
| N2 type | Human: CD11b+, CD66b+, CD170high, PD-L1-; Mouse: CD11b+, CD170high, Ly6G+, PD-L1+ | Tumor growth; Tumor metastasis; Angiogenesis; Immunosuppression |
| NISG | Human: CD66b+, CD11b+, IFIT1, IRF7, RSAD2; Mouse: Ly6G+, CD11b+, IFIT1, IRF7, RSAD2 | Antiviral effect; Tumor immunity |
Tab. 2
TANs-related breast cancer treatment targets and mechanisms"
| Therapeutic mechanism | Target | Drug | Reference |
|---|---|---|---|
| ⑴ Inhibit the infiltration of TANs | CXCR2 | Navarixin (CXCR2 inhibitor) | [ |
| IL-8 | SB225002 (IL-8 inhibitor) | [ | |
| SIRT1-Naged-NETs | [ | ||
| Acod1 | [ | ||
| ⑵ Induce TANs polarization towards the N1 phenotype | TGF-β | SB525334 (TGF-β inhibitor) | [ |
| IFN-β | IFN-βactivator | [ | |
| FATP2 | Lipofermata (FATP2 inhibitor) | [ | |
| E2 | MPP | [ | |
| ⑶ Inhibition of PD-1/PD-L1 checkpoint | Chi3l1 | [ |
| [1] | NÉMETH T, SPERANDIO M, MÓCSAI A. Neutrophils as emerging therapeutic targets[J]. Nat Rev Drug Discov, 2020, 19(4): 253-275. |
| [2] |
JAILLON S, PONZETTA A, MITRI D D, et al. Neutrophil diversity and plasticity in tumour progression and therapy[J]. Nat Rev Cancer, 2020, 20(9): 485-503.
doi: 10.1038/s41568-020-0281-y pmid: 32694624 |
| [3] | CHEN S, ZHANG Q Y, LU L S, et al. Heterogeneity of neutrophils in cancer: one size does not fit all[J]. Cancer Biol Med, 2022, 19(12): 1629-1648. |
| [4] |
BARRY S T, GABRILOVICH D I, SANSOM O J, et al. Therapeutic targeting of tumour myeloid cells[J]. Nat Rev Cancer, 2023, 23(4): 216-237.
doi: 10.1038/s41568-022-00546-2 pmid: 36747021 |
| [5] | SUNG H, FERLAY J, SIEGEL R L, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2021, 71(3): 209-249. |
| [6] | SOTO-PEREZ-DE-CELIS E, CHAVARRI-GUERRA Y, LEON-RODRIGUEZ E, et al. Tumor-associated neutrophils in breast cancer subtypes[J]. Asian Pac J Cancer Prev, 2017, 18(10): 2689-2693. |
| [7] |
HIDALGO A, CHILVERS E R, SUMMERS C, et al. The neutrophil life cycle[J]. Trends Immunol, 2019, 40(7): 584-597.
doi: S1471-4906(19)30102-4 pmid: 31153737 |
| [8] |
FURZE R C, RANKIN S M. Neutrophil mobilization and clearance in the bone marrow[J]. Immunology, 2008, 125(3): 281-288.
doi: 10.1111/j.1365-2567.2008.02950.x pmid: 19128361 |
| [9] | LEY K, HOFFMAN H M, KUBES P, et al. Neutrophils: new insights and open questions[J]. Sci Immunol, 2018, 3(30): eaat4579. |
| [10] |
ADROVER J M, DEL FRESNO C, CRAINICIUC G, et al. A neutrophil timer coordinates immune defense and vascular protection[J]. Immunity, 2019, 51(5): 966-967.
doi: S1074-7613(19)30459-5 pmid: 31747583 |
| [11] | LOH W, VERMEREN S. Anti-inflammatory neutrophil functions in the resolution of inflammation and tissue repair[J]. Cells, 2022, 11(24): 4076. |
| [12] |
ALLEN S J, CROWN S E, HANDEL T M. Chemokine: receptor structure, interactions, and antagonism[J]. Annu Rev Immunol, 2007, 25: 787-820.
pmid: 17291188 |
| [13] | FOUSEK K, HORN L A, PALENA C. Interleukin-8: a chemokine at the intersection of cancer plasticity, angiogenesis, and immune suppression[J]. Pharmacol Ther, 2021, 219: 107692. |
| [14] | HE K, LIU X, HOFFMAN R D, et al. G-CSF/GM-CSF-induced hematopoietic dysregulation in the progression of solid tumors[J]. FEBS Open Bio, 2022, 12(7): 1268-1285. |
| [15] |
XU Y L, YAN J X, TAO Y, et al. Pituitary hormone α-MSH promotes tumor-induced myelopoiesis and immunosuppression[J]. Science, 2022, 377(6610): 1085-1091.
doi: 10.1126/science.abj2674 pmid: 35926007 |
| [16] |
NOURSHARGH S, RENSHAW S A, IMHOF B A. Reverse migration of neutrophils: where, when, how, and why?[J]. Trends Immunol, 2016, 37(5): 273-286.
doi: S1471-4906(16)00044-2 pmid: 27055913 |
| [17] | CORTEZ-RETAMOZO V, ETZRODT M, NEWTON A, et al. Origins of tumor-associated macrophages and neutrophils[J]. Proc Natl Acad Sci U S A, 2012, 109(7): 2491-2496. |
| [18] | FRIDLENDER Z G, SUN J, KIM S, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN[J]. Cancer Cell, 2009, 16(3): 183-194. |
| [19] |
YU X Y, LI C H, WANG Z J, et al. Neutrophils in cancer: dual roles through intercellular interactions[J]. Oncogene, 2024, 43(16): 1163-1177.
doi: 10.1038/s41388-024-03004-5 pmid: 38472320 |
| [20] |
POETA V M, MASSARA M, CAPUCETTI A, et al. Chemokines and chemokine receptors: new targets for cancer immunotherapy[J]. Front Immunol, 2019, 10: 379.
doi: 10.3389/fimmu.2019.00379 pmid: 30894861 |
| [21] | OHMS M, MÖLLER S, LASKAY T. An attempt to polarize human neutrophils toward N1 and N2 phenotypes in vitro[J]. Front Immunol, 2020, 11: 532. |
| [22] |
LIU S Y, WU W C, DU Y S, et al. The evolution and heterogeneity of neutrophils in cancers: origins, subsets, functions, orchestrations and clinical applications[J]. Mol Cancer, 2023, 22(1): 148.
doi: 10.1186/s12943-023-01843-6 pmid: 37679744 |
| [23] | CASBON A J, REYNAUD D, PARK C, et al. Invasive breast cancer reprograms early myeloid differentiation in the bone marrow to generate immunosuppressive neutrophils[J]. Proc Natl Acad Sci U S A, 2015, 112(6): E566-E575. |
| [24] | QUE H Y, FU Q M, LAN T X, et al. Tumor-associated neutrophils and neutrophil-targeted cancer therapies[J]. Biochim Biophys Acta Rev Cancer, 2022, 1877(5): 188762. |
| [25] | LIANG W, LI Q, FERRARA N. Metastatic growth instructed by neutrophil-derived transferrin[J]. Proc Natl Acad Sci U S A, 2018, 115(43): 11060-11065. |
| [26] |
ZHAO Y, LIU Z S, LIU G Q, et al. Neutrophils resist ferroptosis and promote breast cancer metastasis through aconitate decarboxylase 1[J]. Cell Metab, 2023, 35(10): 1688-1703.e10.
doi: 10.1016/j.cmet.2023.09.004 pmid: 37793345 |
| [27] | MA T, TANG Y, WANG T L, et al. Chronic pulmonary bacterial infection facilitates breast cancer lung metastasis by recruiting tumor-promoting MHCⅡhi neutrophils[J]. Signal Transduct Target Ther, 2023, 8(1): 296. |
| [28] | LI P S, LU M, SHI J Y, et al. Lung mesenchymal cells elicit lipid storage in neutrophils that fuel breast cancer lung metastasis[J]. Nat Immunol, 2020, 21(11): 1444-1455. |
| [29] | NG M S F, KWOK I, TAN L, et al. Deterministic reprogramming of neutrophils within tumors[J]. Science, 2024, 383(6679): eadf6493. |
| [30] | LI T J, JIANG Y M, HU Y F, et al. Interleukin-17-producing neutrophils link inflammatory stimuli to disease progression by promoting angiogenesis in gastric cancer[J]. Clin Cancer Res, 2017, 23(6): 1575-1585. |
| [31] | LIU Z L, CHEN H H, ZHENG L L, et al. Angiogenic signaling pathways and anti-angiogenic therapy for cancer[J]. Signal Transduct Target Ther, 2023, 8(1): 198. |
| [32] |
KWANTWI L B, WANG S J, ZHANG W J, et al. Tumor-associated neutrophils activated by tumor-derived CCL20 (C-C motif chemokine ligand 20) promote T cell immunosuppression via programmed death-ligand 1 (PD-L1) in breast cancer[J]. Bioengineered, 2021, 12(1): 6996-7006.
doi: 10.1080/21655979.2021.1977102 pmid: 34519637 |
| [33] |
SPIEGEL A, BROOKS M W, HOUSHYAR S, et al. Neutrophils suppress intraluminal NK cell-mediated tumor cell clearance and enhance extravasation of disseminated carcinoma cells[J]. Cancer Discov, 2016, 6(6): 630-649.
doi: 10.1158/2159-8290.CD-15-1157 pmid: 27072748 |
| [34] | GONG Z, LI Q, SHI J Y, et al. Immunosuppressive reprogramming of neutrophils by lung mesenchymal cells promotes breast cancer metastasis[J]. Sci Immunol, 2023, 8(80): eadd5204. |
| [35] | SHAO B Z, YAO Y, LI J P, et al. The role of neutrophil extracellular traps in cancer[J]. Front Oncol, 2021, 11: 714357. |
| [36] |
MASUCCI M T, MINOPOLI M, DEL VECCHIO S, et al. The emerging role of neutrophil extracellular traps (NETs) in tumor progression and metastasis[J]. Front Immunol, 2020, 11: 1749.
doi: 10.3389/fimmu.2020.01749 pmid: 33042107 |
| [37] | ZHANG Y, GUO L P, DAI Q C, et al. A signature for pan-cancer prognosis based on neutrophil extracellular traps[J]. J Immunother Cancer, 2022, 10(6): e004210. |
| [38] |
HSU B E, TABARIÈS S, JOHNSON R M, et al. Immature low-density neutrophils exhibit metabolic flexibility that facilitates breast cancer liver metastasis[J]. Cell Rep, 2019, 27(13): 3902-3915.e6.
doi: S2211-1247(19)30730-2 pmid: 31242422 |
| [39] |
WANG Y G, DING Y X, GUO N Z, et al. MDSCs: key criminals of tumor pre-metastatic niche formation[J]. Front Immunol, 2019, 10: 172.
doi: 10.3389/fimmu.2019.00172 pmid: 30792719 |
| [40] |
TEIJEIRA Á, GARASA S, GATO M, et al. CXCR1 and CXCR2 chemokine receptor agonists produced by tumors induce neutrophil extracellular traps that interfere with immune cytotoxicity[J]. Immunity, 2020, 52(5): 856-871.e8.
doi: S1074-7613(20)30089-3 pmid: 32289253 |
| [41] |
TAIFOUR T, ATTALLA S S, ZUO D M, et al. The tumor-derived cytokine Chi3l1 induces neutrophil extracellular traps that promote T cell exclusion in triple-negative breast cancer[J]. Immunity, 2023, 56(12): 2755-2772.e8.
doi: 10.1016/j.immuni.2023.11.002 pmid: 38039967 |
| [42] |
MOUSSET A, LECORGNE E, BOURGET I, et al. Neutrophil extracellular traps formed during chemotherapy confer treatment resistance via TGF-β activation[J]. Cancer Cell, 2023, 41(4): 757-775.e10.
doi: 10.1016/j.ccell.2023.03.008 pmid: 37037615 |
| [43] | SCHEDEL F, MAYER-HAIN S, PAPPELBAUM K I, et al. Evidence and impact of neutrophil extracellular traps in malignant melanoma[J]. Pigment Cell Melanoma Res, 2020, 33(1): 63-73. |
| [44] |
FURUMAYA C, MARTINEZ-SANZ P, BOUTI P, et al. Plasticity in pro- and anti-tumor activity of neutrophils: Shifting the balance[J]. Front Immunol, 2020, 11: 2100.
doi: 10.3389/fimmu.2020.02100 pmid: 32983165 |
| [45] |
GERSHKOVITZ M, CASPI Y, FAINSOD-LEVI T, et al. TRPM2 mediates neutrophil killing of disseminated tumor cells[J]. Cancer Res, 2018, 78(10): 2680-2690.
doi: 10.1158/0008-5472.CAN-17-3614 pmid: 29490946 |
| [46] | AGGARWAL V, TULI H S, VAROL A, et al. Role of reactive oxygen species in cancer progression: molecular mechanisms and recent advancements[J]. Biomolecules, 2019, 9(11): 735. |
| [47] |
DENG D, SHAH K. TRAIL of hope meeting resistance in cancer[J]. Trends Cancer, 2020, 6(12): 989-1001.
doi: 10.1016/j.trecan.2020.06.006 pmid: 32718904 |
| [48] |
CUI C, CHAKRABORTY K, TANG X A, et al. Neutrophil elastase selectively kills cancer cells and attenuates tumorigenesis[J]. Cell, 2021, 184(12): 3163-3177.e21.
doi: 10.1016/j.cell.2021.04.016 pmid: 33964209 |
| [49] |
PONZETTA A, CARRIERO R, CARNEVALE S, et al. Neutrophils driving unconventional T cells mediate resistance against murine sarcomas and selected human tumors[J]. Cell, 2019, 178(2): 346-360.e24.
doi: S0092-8674(19)30618-X pmid: 31257026 |
| [50] |
WU Y C, MA J Q, YANG X P, et al. Neutrophil profiling illuminates anti-tumor antigen-presenting potency[J]. Cell, 2024, 187(6): 1422-1439.e24.
doi: 10.1016/j.cell.2024.02.005 pmid: 38447573 |
| [51] | KAKUMOTO A, JAMIYAN T, KURODA H, et al. Prognostic impact of tumor-associated neutrophils in breast cancer[J]. Int J Clin Exp Pathol, 2024, 17(3): 51-62. |
| [52] |
PU N, YIN H L, ZHAO G C, et al. Independent effect of postoperative neutrophil-to-lymphocyte ratio on the survival of pancreatic ductal adenocarcinoma with open distal pancreatosplenectomy and its nomogram-based prediction[J]. J Cancer, 2019, 10(24): 5935-5943.
doi: 10.7150/jca.35856 pmid: 31762803 |
| [53] |
HUANG Z N, LI Z X, YAO Z C, et al. Clinical prognostic evaluation of immunocytes in different molecular subtypes of breast cancer[J]. J Cell Physiol, 2019, 234(11): 20584-20602.
doi: 10.1002/jcp.28662 pmid: 31016756 |
| [54] | VON AU A, SHENCORU S, UHLMANN L, et al. Predictive value of neutrophil-to-lymphocyte-ratio in neoadjuvant-treated patients with breast cancer[J]. Arch Gynecol Obstet, 2023, 307(4): 1105-1113. |
| [55] | ZHANG X, GE X, JIANG T, et al. Research progress on immunotherapy in triple-negative breast cancer (Review)[J]. Int J Oncol, 2022, 61(2): 95. |
| [56] | HE J, ZHOU M X, YIN J, et al. METTL3 restrains papillary thyroid cancer progression via m6A/c-Rel/IL-8-mediated neutrophil infiltration[J]. Mol Ther, 2021, 29(5): 1821-1837. |
| [57] | LIN Q, FANG X L, LIANG G H, et al. Silencing CTNND1 mediates triple-negative breast cancer bone metastasis via upregulating CXCR4/CXCL12 axis and neutrophils infiltration in bone[J]. Cancers, 2021, 13(22): 5703. |
| [58] | YANG C H, WANG Z, LI L L, et al. Aged neutrophils form mitochondria-dependent vital NETs to promote breast cancer lung metastasis[J]. J Immunother Cancer, 2021, 9(10): e002875. |
| [59] | PENG H M, SHEN J, LONG X, et al. Local release of TGF-β inhibitor modulates tumor-associated neutrophils and enhances pancreatic cancer response to combined irreversible electroporation and immunotherapy[J]. Adv Sci, 2022, 9(10): e2105240. |
| [60] |
YU R R, ZHU B, CHEN D G. Type I interferon-mediated tumor immunity and its role in immunotherapy[J]. Cell Mol Life Sci, 2022, 79(3): 191.
doi: 10.1007/s00018-022-04219-z pmid: 35292881 |
| [61] | VEGLIA F, TYURIN V A, BLASI M, et al. Fatty acid transport protein 2 reprograms neutrophils in cancer[J]. Nature, 2019, 569(7754): 73-78. |
| [62] |
LINDE I L, PRESTWOOD T R, QIU J T, et al. Neutrophil-activating therapy for the treatment of cancer[J]. Cancer Cell, 2023, 41(2): 356-372.e10.
doi: 10.1016/j.ccell.2023.01.002 pmid: 36706760 |
| [63] |
VAZQUEZ RODRIGUEZ G, ABRAHAMSSON A, JENSEN L D, et al. Estradiol promotes breast cancer cell migration via recruitment and activation of neutrophils[J]. Cancer Immunol Res, 2017, 5(3): 234-247.
doi: 10.1158/2326-6066.CIR-16-0150 pmid: 28159748 |
| [64] | MINOR B M N, LEMOINE D, SEGER C, et al. Estradiol augments tumor-induced neutrophil production to promote tumor cell actions in lymphangioleiomyomatosis models[J]. Endocrinology, 2023, 164(6): bqad061. |
| [65] | DAASSI D, MAHONEY K M, FREEMAN G J. The importance of exosomal PD-L1 in tumour immune evasion[J]. Nat Rev Immunol, 2020, 20(4): 209-215. |
| [66] | WANG T T, ZHAO Y L, PENG L S, et al. Tumour-activated neutrophils in gastric cancer foster immune suppression and disease progression through GM-CSF-PD-L1 pathway[J]. Gut, 2017, 66(11): 1900-1911. |
| [67] | KHOU S, POPA A, LUCI C, et al. Tumor-associated neutrophils dampen adaptive immunity and promote cutaneous squamous cell carcinoma development[J]. Cancers, 2020, 12(7): 1860. |
| [68] | ZHAO Y, BAI Y S, SHEN M L, et al. Therapeutic strategies for gastric cancer targeting immune cells: future directions[J]. Front Immunol, 2022, 13: 992762. |
| [69] | SUN R, XIONG Y Y, LIU H J, et al. Tumor-associated neutrophils suppress antitumor immunity of NK cells through the PD-L1/PD-1 axis[J]. Transl Oncol, 2020, 13(10): 100825. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
沪ICP备12009617
Powered by Beijing Magtech Co. Ltd
