Welcome to China Oncology,
China Oncology ›› 2022, Vol. 32 ›› Issue (12): 1190-1198.doi: 10.19401/j.cnki.1007-3639.2022.12.007
• Article • Previous Articles Next Articles
WU Quan( ), GUO Jingwei, LEI Yuxin, HU Xiaoru, WANG Zhe(
), GUO Jingwei, LEI Yuxin, HU Xiaoru, WANG Zhe( )
)
Received:2022-05-13
Revised:2022-11-08
Online:2022-12-30
Published:2023-02-02
Contact:
WANG Zhe
Share article
CLC Number:
WU Quan, GUO Jingwei, LEI Yuxin, HU Xiaoru, WANG Zhe. Expression of MMR in 515 cases of endometrioid adenocarcinoma and its correlation with clinicopathological features[J]. China Oncology, 2022, 32(12): 1190-1198.
Fig. 1
The expression of MMR protein and p53 protein in endometrioid adenocarcinoma A-E: H-E and IHC staining in the same case of endometrial cancer (×25); A: H-E staining of well differentiated endometrioid adenocarcinoma; B: MLH1 protein was positively expressed in tumor nuclei; C: PMS2 protein was positively expressed in tumor nuclei; D: MSH2 protein was positively expressed in tumor nuclei; E: MSH6 protein was positively expressed in tumor nuclei. F-H: H-E and IHC staining in the same case of endometrial cancer (×100); F: H-E staining of moderately differentiated endometrioid adenocarcinoma; G: The expression of MLH1 protein was negative in tumor nucleus and positive in interstitial nucleus; H: The expression of PMS2 protein was negative in tumor nucleus, weakly positive in tumor cell membrane and positive in interstitial nucleus. I-L: H-E and IHC staining in the same case of endometrial cancer (×100); I: H-E staining of well differentiated endometrioid adenocarcinoma; J: The expression of MSH2 protein was negative in tumor nucleus; K: The expression of MSH6 protein was negative in tumor nucleus and positive in interstitial nucleus; L: Abnormal expression of p53 protein, the expression of p53 protein was negative in tumor nucleus."
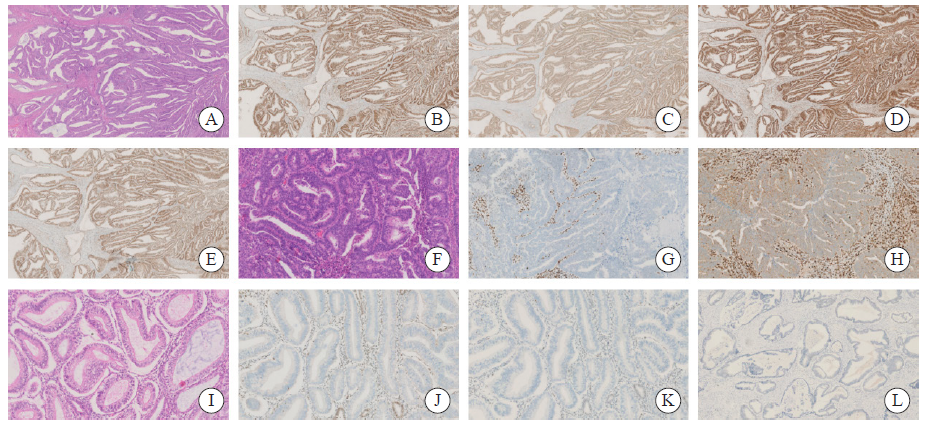
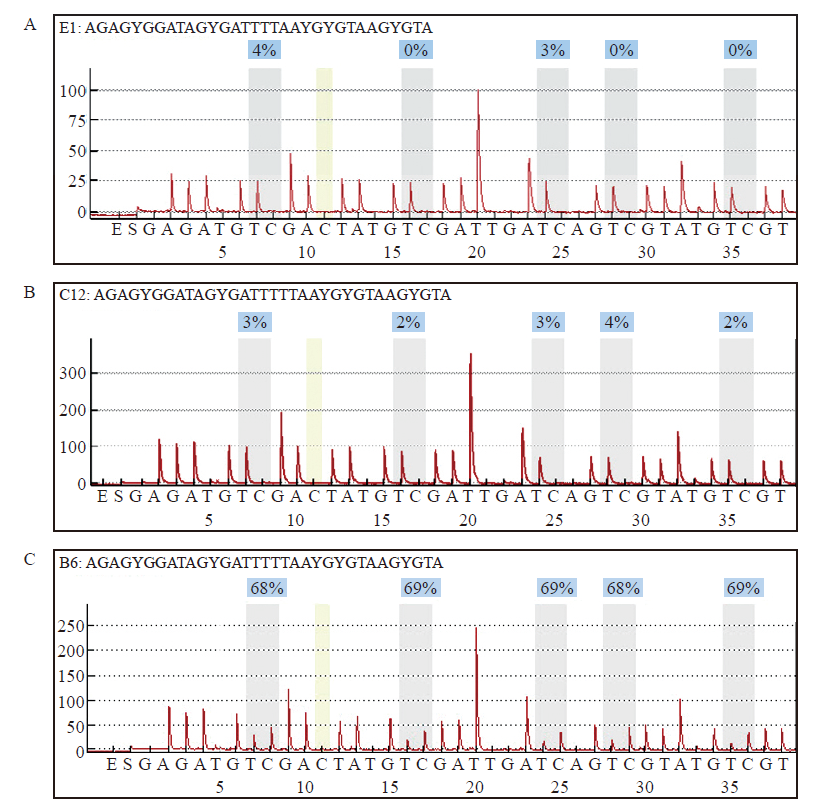
Fig. 3
MLH1 methylation was detected in sample with protein expression deletion A: Reference map of normal samples; B: MLH1 methylation was negative in dMMR endometrioid adenocarcinoma with MLH1 protein expression deletion; C: MLH1 methylation was positive in dMMR endometrioid adenocarcinoma with MLH1 protein expression deletion."
Tab. 1
Correlation between MMR protein expression loss and clinicopathological features"
| Characteristics | Overall population | dMMR | Mismatch repair protein | |||
|---|---|---|---|---|---|---|
| MLH1 | MSH2 | MSH6 | PMS2 | |||
| Age/year | ||||||
| <60 | 303 | 96 (31.7)* | 56 (18.5) | 23 (7.6)* | 31 (10.2)* | 65 (21.5) |
| ≥60 | 212 | 42 (19.8) | 28 (13.2) | 5 (2.4) | 10 (4.7) | 33 (15.6) |
| FIGO stage | ||||||
| Ⅰ-Ⅱ | 466 | 117 (25.1) | 72 (15.5) | 22 (4.7) | 35 (7.5) | 84 (18.0) |
| Ⅲ-Ⅳ | 49 | 21 (42.9)* | 12 (24.5) | 6 (12.2)* | 6 (12.2) | 14 (28.6) |
| The organizational differentiation | ||||||
| Well and moderately differentiated | 473 | 115 (24.3) | 82 (17.3) | 23 (4.8) | 36 (7.6) | 82 (17.3) |
| Poorly differentiated | 39 | 23 (59.0)* | 13 (33.3)* | 4 (10.2) | 5 (12.8) | 16 (41.0)* |
| The myometrial infiltration | ||||||
| Positive | 451 | 114 (25.3) | 72 (16.0) | 20 (4.4) | 30 (6.7) | 84 (18.6) |
| Negative | 64 | 24 (37.5)* | 12 (18.8) | 8 (12.5)* | 11 (17.2)* | 14 (21.9) |
| The lymph node metastasis | ||||||
| Positive | 42 | 19 (45.2)* | 12 (28.6)* | 4 (9.5) | 4 (9.5) | 14 (33.3)* |
| Negative | 473 | 119 (25.2) | 72 (15.2) | 24 (5.1) | 37 (7.8) | 84 (17.8) |
| p53 expression | ||||||
| Abnormal | 138 | 29 (21.0) | 24 (17.4) | 5 (3.6) | 5 (3.6) | 26 (18.8) |
| Normal | 372 | 107 (28.8) | 58 (15.6) | 23 (6.2) | 36 (9.7)* | 70 (18.8) |
| The lower uterine segment involved | ||||||
| Positive | 74 | 22 (29.7) | 16 (21.6) | 6 (8.1) | 5 (6.8) | 17 (23.0) |
| Negative | 441 | 116 (26.3) | 68 (15.4) | 22 (5.0) | 36 (8.2) | 81 (18.4) |
| The vascular metastasis | ||||||
| Positive | 18 | 12 (66.7)* | 6 (33.3) | 3 (16.7) | 3 (16.7) | 8 (44.4) |
| Negative | 35 | 10 (28.6) | 7 (20.0) | 2 (5.7) | 2 (5.7) | 8 (22.9) |
| The nerve invasion | ||||||
| Positive | 20 | 13 (65.0)* | 7 (35.0) | 3 (15.0) | 3 (15.0) | 9 (45.0) |
| Negative | 34 | 9 (26.5) | 6 (17.6) | 2 (5.9) | 2 (5.9) | 7 (20.6) |
| Tumor infiltrating lymphocytes | ||||||
| ≥42 | 130 | 64 (49.2)* | 41 (31.5)* | 13 (10.0)* | 17 (13.1)* | 47 (36.2)* |
| <42 | 226 | 30 (13.3) | 20 (8.8) | 5 (2.2) | 9 (4.0) | 23 (10.2) |
| Tumor with peritumoral lymphocyte infiltration | ||||||
| Positive | 201 | 80 (39.8)* | 52 (25.9)* | 14 (7.0) | 22 (10.9)* | 61 (30.3)* |
| Negative | 155 | 15 (9.7) | 9 (5.8) | 4 (2.6) | 4 (2.6) | 9 (5.8) |
| [1] |
BELL D W, ELLENSON L H. Molecular genetics of endometrial carcinoma[J]. Annu Rev Pathol, 2019, 14: 339-367.
doi: 10.1146/annurev-pathol-020117-043609 pmid: 30332563 |
| [2] |
LYNCH H T, LYNCH P M, LANSPA S J, et al. Review of the Lynch syndrome: history, molecular genetics, screening, differential diagnosis, and medicolegal ramifications[J]. Clin Genet, 2009, 76(1): 1-18.
doi: 10.1111/j.1399-0004.2009.01230.x pmid: 19659756 |
| [3] |
LU K H, SCHORGE J O, RODABAUGH K J, et al. Prospective determination of prevalence of lynch syndrome in young women with endometrial cancer[J]. J Clin Oncol, 2007, 25(33): 5158-5164.
pmid: 17925543 |
| [4] |
YAMAMOTO H, IMAI K. Microsatellite instability: an update[J]. Arch Toxicol, 2015, 89(6): 899-921.
doi: 10.1007/s00204-015-1474-0 pmid: 25701956 |
| [5] | DE' ANGELIS GL, BOTTARELLI L, AZZONI C, et al. Microsatellite instability in colorectal cancer[J]. Acta Biomed, 2018, 89(9s): 97-101. |
| [6] |
SHIA, BLACK D, HUMMER A J, et al. Routinely assessed morphological features correlate with microsatellite instability status in endometrial cancer[J]. Hum Pathol, 2008, 39(1): 116-125.
pmid: 17949789 |
| [7] |
KWON J S, SUN C C, PETERSON S K, et al. Cost-effectiveness analysis of prevention strategies for gynecologic cancers in Lynch syndrome[J]. Cancer, 2008, 113(2): 326-335.
doi: 10.1002/cncr.23554 pmid: 18506736 |
| [8] | CHAO X P, LI L, WU M, et al. Comparison of screening strategies for Lynch syndrome in patients with newly diagnosed endometrial cancer: a prospective cohort study in China[J]. Cancer Commun (Lond), 2019, 39(1): 42. |
| [9] |
SHENG J Q, FU L, SUN Z Q, et al. Mismatch repair gene mutations in Chinese HNPCC patients[J]. Cytogenet Genome Res, 2008, 122(1): 22-27.
doi: 10.1159/000151312 pmid: 18931482 |
| [10] |
MANCHANA T, ARIYASRIWATANA C, TRIRATANACHAT S, et al. Lynch syndrome in Thai endometrial cancer patients[J]. Asian Pac J Cancer Prev, 2021, 22(5): 1477-1483.
doi: 10.31557/APJCP.2021.22.5.1477 |
| [11] | 晋薇, 王利群, 刘有, 等. 子宫内膜癌组织中MMR蛋白表达及MLH1基因甲基化的临床意义[J]. 中华妇产科杂志, 2018, 53(12): 823-830. |
| JIN W, WANG L Q, LIU Y, et al. Expression and clinical significance of MMR protein and MLH1 promoter methylation testing in endometrial cancer[J]. Chin J Obstet Gynecol, 2018, 53(12): 823-830. | |
| [12] |
ZHAO S S, CHEN L L, ZANG Y Q, et al. Endometrial cancer in Lynch syndrome[J]. Int J Cancer, 2022, 150(1): 7-17.
doi: 10.1002/ijc.33763 |
| [13] |
RYAN N A J, MORRIS J, GREEN K, et al. Association of mismatch repair mutation with age at cancer onset in Lynch syndrome: implications for stratified surveillance strategies[J]. JAMA Oncol, 2017, 3(12): 1702-1706.
doi: 10.1001/jamaoncol.2017.0619 pmid: 28772289 |
| [14] | BI R, TU X Y, XIAO Y X, et al. Clinicopathological analysis of the expression of mismatch repair protein in endometrial carcinoma[J]. Chin J Pathol, 2016, 45(5): 302-307. |
| [15] |
MCMEEKIN D S, TRITCHLER D L, COHN D E, et al. Clinicopathologic significance of mismatch repair defects in endometrial cancer: an NRG oncology/gynecologic oncology group study[J]. J Clin Oncol, 2016, 34(25): 3062-3068.
doi: 10.1200/JCO.2016.67.8722 pmid: 27325856 |
| [16] |
WORKEL H H, KOMDEUR F L, WOUTERS M C, et al. CD103 defines intraepithelial CD8+ PD1+ tumour-infiltrating lymphocytes of prognostic significance in endometrial adenocarcinoma[J]. Eur J Cancer, 2016, 60: 1-11.
doi: 10.1016/j.ejca.2016.02.026 |
| [17] |
SHIKAMA A, MINAGUCHI T, MATSUMOTO K, et al. Clinicopathologic implications of DNA mismatch repair status in endometrial carcinomas[J]. Gynecol Oncol, 2016, 140(2): 226-233.
doi: 10.1016/j.ygyno.2015.11.032 pmid: 26644264 |
| [18] |
SIEGEL R L, MILLER K D, JEMAL A. Cancer statistics, 2015[J]. CA Cancer J Clin, 2015, 65(1): 5-29.
doi: 10.3322/caac.21254 |
| [19] |
HOANG L N, KINLOCH M A, LEO J M, et al. Interobserver agreement in endometrial carcinoma histotype diagnosis varies depending on the Cancer Genome Atlas (TCGA)-based molecular subgroup[J]. Am J Surg Pathol, 2017, 41(2): 245-252.
doi: 10.1097/PAS.0000000000000764 pmid: 28079598 |
| [20] |
LEÓN-CASTILLO A, DE BOER S M, POWELL M E, et al. Molecular classification of the PORTEC-3 trial for high-risk endometrial cancer: impact on prognosis and benefit from adjuvant therapy[J]. J Clin Oncol, 2020, 38(29): 3388-3397.
doi: 10.1200/JCO.20.00549 |
| [21] | WILLIAMS A B, SCHUMACHER B. p53 in the DNA-damage-repair process[J]. Cold Spring Harb Perspect Med, 2016, 6(5): a026070. |
| [22] |
CRANSTON A, BOCKER T, REITMAIR A, et al. Female embryonic lethality in mice nullizygous for both MSH2 and p53[J]. Nat Genet, 1997, 17(1): 114-118.
pmid: 9288110 |
| [23] |
SUBRAMANIAN D, GRIFFITH J D. Modulation of p53 binding to holliday junctions and 3-cytosine bulges by phosphorylation events[J]. Biochemistry, 2005, 44(7): 2536-2544.
doi: 10.1021/bi048700u pmid: 15709766 |
| [24] |
GUILLOTIN D, MARTIN S A. Exploiting DNA mismatch repair deficiency as a therapeutic strategy[J]. Exp Cell Res, 2014, 329(1): 110-115.
doi: 10.1016/j.yexcr.2014.07.004 pmid: 25017099 |
| [25] |
FRANCHITTO A, PICHIERRI P, PIERGENTILI R, et al. The mammalian mismatch repair protein MSH2 is required for correct MRE11 and RAD51 relocalization and for efficient cell cycle arrest induced by ionizing radiation in G2 phase[J]. Oncogene, 2003, 22(14): 2110-2120.
doi: 10.1038/sj.onc.1206254 |
| [26] |
REIJNEN C, KÜSTERS-VANDEVELDE H V N, PRINSEN C F, et al. Mismatch repair deficiency as a predictive marker for response to adjuvant radiotherapy in endometrial cancer[J]. Gynecol Oncol, 2019, 154(1): 124-130.
doi: S0090-8258(19)30270-7 pmid: 31103324 |
| [27] |
LYNCH H T, SNYDER C L, SHAW T G, et al. Milestones of lynch syndrome: 1895-2015[J]. Nat Rev Cancer, 2015, 15(3): 181-194.
doi: 10.1038/nrc3878 pmid: 25673086 |
| [28] |
KLOOR M, BECKER C, BENNER A, et al. Immunoselective pressure and human leukocyte antigen class Ⅰ antigen machinery defects in microsatellite unstable colorectal cancers[J]. Cancer Res, 2005, 65(14): 6418-6424.
doi: 10.1158/0008-5472.CAN-05-0044 |
| [29] |
BURN J, BISHOP D T, MECKLIN J P, et al. Effect of aspirin or resistant starch on colorectal neoplasia in the Lynch syndrome[J]. N Engl J Med, 2008, 359(24): 2567-2578.
doi: 10.1056/NEJMoa0801297 |
| [30] |
PARDOLL D M. The blockade of immune checkpoints in cancer immunotherapy[J]. Nat Rev Cancer, 2012, 12(4): 252-264.
doi: 10.1038/nrc3239 pmid: 22437870 |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
沪ICP备12009617
Powered by Beijing Magtech Co. Ltd
