Welcome to China Oncology,
China Oncology ›› 2023, Vol. 33 ›› Issue (7): 693-700.doi: 10.19401/j.cnki.1007-3639.2023.07.007
• Article • Previous Articles Next Articles
WANG Yan1( ), SU Yue1, HU Tu2, LIU Qiying3, YAO Weiqiang1, CHEN Yong2, YAN Wangjun2, ZHANG Zhen1(
), SU Yue1, HU Tu2, LIU Qiying3, YAO Weiqiang1, CHEN Yong2, YAN Wangjun2, ZHANG Zhen1( )
)
Received:2023-02-02
Revised:2023-06-29
Online:2023-07-30
Published:2023-08-10
Contact:
ZHANG Zhen.
Share article
CLC Number:
WANG Yan, SU Yue, HU Tu, LIU Qiying, YAO Weiqiang, CHEN Yong, YAN Wangjun, ZHANG Zhen. Retrospective analysis of short-term efficacy and safety of preoperative radiotherapy for 33 cases of locally high-risk soft tissue sarcoma[J]. China Oncology, 2023, 33(7): 693-700.
Tab. 1
Clinical characteristics of patients [n (%)]"
| Characteristic | Case |
|---|---|
| Gender | |
| Male | 17 (51.5) |
| Female | 16 (48.5) |
| Age/year | |
| <60 | 20 (60.6) |
| ≥60 | 13 (39.4) |
| locations of tumor | |
| Head and neck | 3 (9.1) |
| Trunk | 11 (33.3) |
| Upper extremity | 3 (9.1) |
| Lower extremity | 15 (45.5) |
| Retroperitoneum | 1 (3.0) |
| Largest tumor dimension/cm | |
| <5 | 6 (18.2) |
| 5-10 | 14 (42.4) |
| ≥10 | 13 (39.4) |
| Previous treatments | |
| Primary | 13 (39.4) |
| Recurrence/metastasis | |
| Previous surgery alone | 15 (45.5) |
| Previous chemotherapy alone | 1 (3.0) |
| Previous surgery and chemotherapy | 4 (12.1) |
| Concurrent treatment | |
| Chemotherapy | 15 (45.4) |
| Target therapy | 3 (9.1) |
| Chemotherapy and target therapy | 1 (3.0) |
| Target therapy and immunotherapy | 2 (6.1) |
| No | 12 (36.4) |
Tab. 2
Pathologic characteristics of the patients [n (%)]"
| Pathologic subtype | Case |
|---|---|
| Rhabdomyosarcoma | 4 (12.1) |
| Leiomyosarcoma | 1 (3.0) |
| Fibroblastic and myofibroblastic tumors | |
| Myxofibrosarcoma | 3 (9.1) |
| Others | 3 (9.1) |
| Angiosarcoma | 1 (3.0) |
| Peripheral nerve sheath tumors | |
| Malignant peripheral nerve sheath tumor | 1 (3.0) |
| Malignant triton tumor | 1 (3.0) |
| Others | 1 (3.0) |
| Liposarcoma | |
| Atypical lipomatous tumor/well-differentiated liposarcoma | 1 (3.0) |
| Dedifferentiated liposarcoma | 1 (3.0) |
| Pleomorphic liposarcoma | 1 (3.0) |
| Tumors of uncertain differentiation | |
| Synovial sarcoma | 4 (12.1) |
| epithelioid sarcom | 1 (3.0) |
| Undifferentiated pleomorphic sarcoma | 5 (15.2) |
| Round cell sarcoma | |
| Extraskeletal Ewing’s sarcoma | 1 (3.0) |
| Others | 3 (9.1) |
| Spindle cell sarcoma | 1 (3.0) |
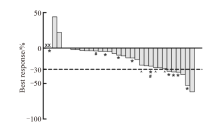
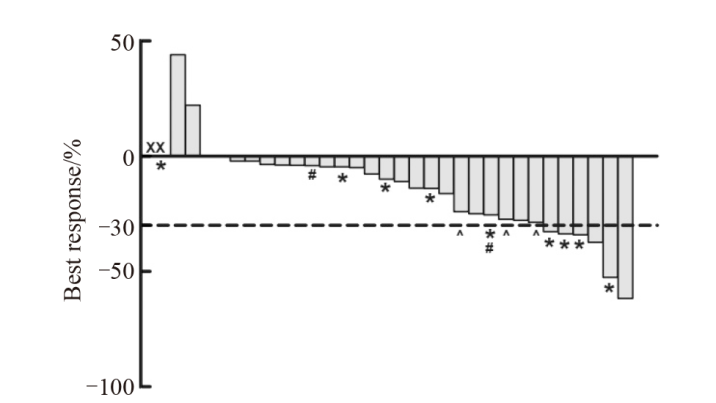
Fig. 2
Efficacy of preoperative radiotherapy Plot shows dimensional change in target lesions as a percentage of the baseline measurement. Lower dashed lines represent the cutoffs for partial response (30% decrease in the sum of diameters of target lesions). X: Lost the reexamination images after radiotherapy; *: Near-pCR; #: Received delayed surgery; ^: Refused surgery or lost to follow-up."
| [1] |
VON MEHREN M, KANE J M, AGULNIK M, et al. Soft tissue sarcoma, version 2.2022, NCCN clinical practice guidelines in oncology[J]. J Natl Compr Canc Netw, 2022, 20(7): 815-833.
doi: 10.6004/jnccn.2022.0035 |
| [2] | KAINHOFER V, SMOLLE M A, SZKANDERA J, et al. The width of resection margins influences local recurrence in soft tissue sarcoma patients[J]. Eur J Surg Oncol EJSO, 2016, 42(6): 899-906. |
| [3] |
DAVIS A M, O'SULLIVAN B, BELL R S, et al. Function and health status outcomes in a randomized trial comparing preoperative and postoperative radiotherapy in extremity soft tissue sarcoma[J]. J Clin Oncol, 2002, 20(22): 4472-4477.
doi: 10.1200/JCO.2002.03.084 pmid: 12431971 |
| [4] |
SAMPATH S, SCHULTHEISS T E, HITCHCOCK Y J, et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma: multi-institutional analysis of 821 patients[J]. Int J Radiat Oncol Biol Phys, 2011, 81(2): 498-505.
doi: 10.1016/j.ijrobp.2010.06.034 |
| [5] |
DAVIS A, OSULLIVAN B, TURCOTTE R, et al. Late radiation morbidity following randomization to preoperative versus postoperative radiotherapy in extremity soft tissue sarcoma[J]. Radiother Oncol, 2005, 75(1): 48-53.
doi: 10.1016/j.radonc.2004.12.020 pmid: 15948265 |
| [6] |
O’SULLIVAN B, DAVIS A M, TURCOTTE R, et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial[J]. Lancet, 2002, 359(9325): 2235-2241.
doi: 10.1016/S0140-6736(02)09292-9 pmid: 12103287 |
| [7] |
FOLKERT M R, SINGER S, BRENNAN M F, et al. Comparison of local recurrence with conventional and intensity-modulated radiation therapy for primary soft-tissue sarcomas of the extremity[J]. J Clin Oncol, 2014, 32(29): 3236-3241.
doi: 10.1200/JCO.2013.53.9452 pmid: 25185087 |
| [8] |
O’SULLIVAN B, GRIFFIN A M, DICKIE C I, et al. Phase 2 study of preoperative image-guided intensity-modulated radiation therapy to reduce wound and combined modality morbidities in lower extremity soft tissue sarcoma[J]. Cancer, 2013, 119(10): 1878-1884.
doi: 10.1002/cncr.27951 pmid: 23423841 |
| [9] |
LI X A, CHEN X J, ZHANG Q, et al. Margin reduction from image guided radiation therapy for soft tissue sarcoma: secondary analysis of Radiation Therapy Oncology Group 0630 results[J]. Pract Radiat Oncol, 2016, 6(4): e135-e140.
doi: 10.1016/j.prro.2015.11.012 pmid: 26852173 |
| [10] |
WANG D, BOSCH W, ROBERGE D, et al. RTOG sarcoma radiation oncologists reach consensus on gross tumor volume and clinical target volume on computed tomographic images for preoperative radiotherapy of primary soft tissue sarcoma of extremity in Radiation Therapy Oncology Group studies[J]. Int J Radiat Oncol Biol Phys, 2011, 81(4): e525-e528.
doi: 10.1016/j.ijrobp.2011.04.038 |
| [11] |
EISENHAUER E A, THERASSE P, BOGAERTS J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1)[J]. Eur J Cancer, 2009, 45(2): 228-247.
doi: 10.1016/j.ejca.2008.10.026 pmid: 19097774 |
| [12] |
WEISS A R, CHEN Y L, SCHARSCHMIDT T J, et al. Pathological response in children and adults with large unresected intermediate-grade or high-grade soft tissue sarcoma receiving preoperative chemoradiotherapy with or without pazopanib (ARST1321): a multicentre, randomised, open-label, phase 2 trial[J]. Lancet Oncol, 2020, 21(8): 1110-1122.
doi: S1470-2045(20)30325-9 pmid: 32702309 |
| [13] |
ASHLEIGH GUADAGNOLO B, BASSETT R L, MITRA D, et al. Hypofractionated, 3-week, preoperative radiotherapy for patients with soft tissue sarcomas (HYPORT-STS): a single-centre, open-label, single-arm, phase 2 trial[J]. Lancet Oncol, 2022, 23(12): 1547-1557.
doi: 10.1016/S1470-2045(22)00638-6 pmid: 36343656 |
| [14] | National Cancer Institute. Protocol development cancer therapy evaluation program[EB/OL]. (2017-11-27)[2023-04-28]. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm. |
| [15] | COINDRE J M. Recommendations for anatamo-pathologic management of soft tissue sarcomas in the adult. Pathologists of the FNCLCC Sarcoma Group (Fédération Nationale des Centres de Lutte Contre le Cancer)[J]. Ann Pathol, 1998, 18(6): 505-511. |
| [16] |
SBARAGLIA M, BELLAN E, DEI TOS A P. The 2020 WHO Classification of Soft Tissue Tumours: news and perspectives[J]. Pathologica, 2021, 113(2): 70-84.
doi: 10.32074/1591-951X-213 pmid: 33179614 |
| [17] |
FENG X Y, LI J, LI A M, et al. Stereotactic body radiotherapy for recurrent and oligometastatic soft tissue sarcoma[J]. World J Surg Oncol, 2022, 20(1): 322.
doi: 10.1186/s12957-022-02781-1 |
| [18] |
HUANG Z, LI N, TANG Y, et al. Neoadjuvant radiotherapy for soft tissue sarcoma in China: a preliminary result[J]. Ann Transl Med, 2022, 10(8): 452.
doi: 10.21037/atm-22-98 pmid: 35571451 |
| [19] |
CASALI P G, ABECASSIS N, ARO H T, et al. Soft tissue and visceral sarcomas: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up[J]. Ann Oncol, 2018, 29(Suppl 4): iv51-iv67.
doi: 10.1093/annonc/mdy096 |
| [20] |
GORTZAK E, AZZARELLI A, BUESA J, et al. A randomised phase Ⅱ study on neo-adjuvant chemotherapy for ‘high-risk’ adult soft-tissue sarcoma[J]. Eur J Cancer, 2001, 37(9): 1096-1103.
doi: 10.1016/S0959-8049(01)00083-1 |
| [21] |
VOSS R K, CHIANG Y J, TORRES K E, et al. Adherence to national comprehensive cancer network guidelines is associated with improved survival for patients with stage 2A and stages 2B and 3 extremity and superficial trunk soft tissue sarcoma[J]. Ann Surg Oncol, 2017, 24(11): 3271-3278.
doi: 10.1245/s10434-017-6015-z pmid: 28741122 |
| [22] |
SCHAEFER I M, HORNICK J L, BARYSAUSKAS C M, et al. Histologic appearance after preoperative radiation therapy for soft tissue sarcoma: assessment of the European organization for research and treatment of cancer-soft tissue and bone sarcoma group response score[J]. Int J Radiat Oncol Biol Phys, 2017, 98(2): 375-383.
doi: 10.1016/j.ijrobp.2017.02.087 |
| [23] |
TANAKA K, OGAWA G, MIZUSAWA J, et al. Prospective comparison of various radiological response criteria and pathological response to preoperative chemotherapy and survival in operable high-grade soft tissue sarcomas in the Japan Clinical Oncology Group study JCOG0304[J]. World J Surg Oncol, 2018, 16(1): 162.
doi: 10.1186/s12957-018-1462-y pmid: 30097070 |
| [24] |
WAHL R L, JACENE H, KASAMON Y, et al. From RECIST to PERCIST: evolving Considerations for PET response criteria in solid tumors[J]. J Nucl Med, 2009, 50(Suppl 1): 122S-150S.
doi: 10.2967/jnumed.108.057307 |
| [25] |
YANG X, ZHANG L, YANG X, et al. Oncologic outcomes of pre- versus post-operative radiation in Resectable soft tissue sarcoma: a systematic review and meta-analysis[J]. Radiat Oncol, 2020, 15(1): 158.
doi: 10.1186/s13014-020-01600-9 pmid: 32576267 |
| [26] |
KALBASI A, KAMRAVA M, CHU F I, et al. A phase Ⅱ trial of 5-day neoadjuvant radiotherapy for patients with high-risk primary soft tissue sarcoma[J]. Clin Cancer Res, 2020, 26(8): 1829-1836.
doi: 10.1158/1078-0432.CCR-19-3524 |
| [27] |
ALBERTSMEIER M, RAUCH A, ROEDER F, et al. External beam radiation therapy for resectable soft tissue sarcoma: A systematic review and meta-analysis[J]. Ann Surg Oncol, 2018, 25(3): 754-767.
doi: 10.1245/s10434-017-6081-2 pmid: 28895107 |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
沪ICP备12009617
Powered by Beijing Magtech Co. Ltd
