Welcome to China Oncology,
China Oncology ›› 2024, Vol. 34 ›› Issue (1): 97-103.doi: 10.19401/j.cnki.1007-3639.2024.01.007
• Review • Previous Articles Next Articles
LIU Xuerou1,2( ), YANG Yumei1,2, ZHAO Qian1,2, RONG Xiangyu1,2, LIU Wei1,2, ZHENG Ruijie1,2, PANG Jinlong1,2, LI Xian1,2, LI Shanshan1,2(
), YANG Yumei1,2, ZHAO Qian1,2, RONG Xiangyu1,2, LIU Wei1,2, ZHENG Ruijie1,2, PANG Jinlong1,2, LI Xian1,2, LI Shanshan1,2( )
)
Received:2023-10-26
Revised:2023-12-20
Online:2024-01-30
Published:2024-02-05
Contact:
LI Shanshan.
Share article
CLC Number:
LIU Xuerou, YANG Yumei, ZHAO Qian, RONG Xiangyu, LIU Wei, ZHENG Ruijie, PANG Jinlong, LI Xian, LI Shanshan. Research progress on the role of glutamine metabolism-related proteins in tumor metastasis[J]. China Oncology, 2024, 34(1): 97-103.
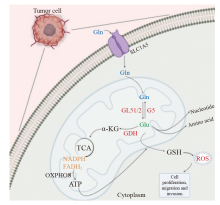
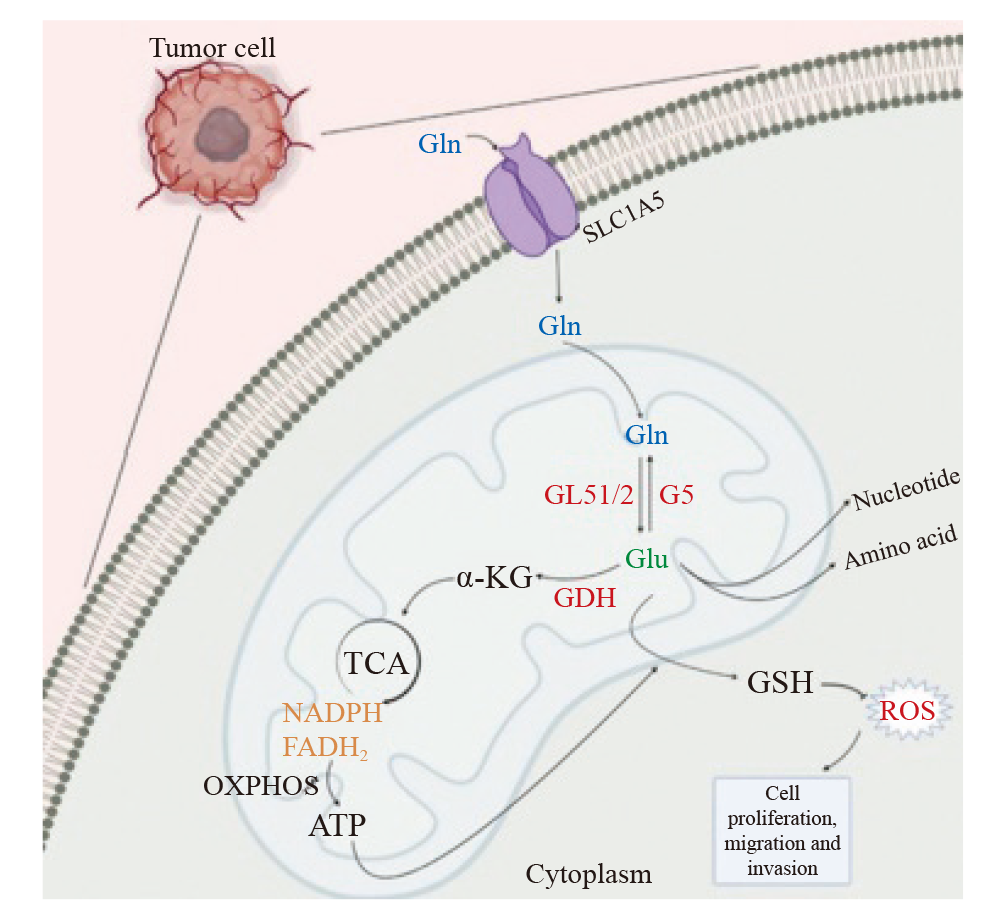
Fig. 1
The main pathway of glutamine metabolism Glutamine enters the mitochondria of tumor cells via SL1A5 and is used to maintain the stability of intracellular redox levels through a series of reactions produce metabolites that affect the proliferation, invasion and migration of tumor cells. ROS: Reactive oxygen species."
| [1] |
REN L, RUIZ-RODADO V, DOWDY T, et al. Glutaminase-1 (GLS1) inhibition limits metastatic progression in osteosarcoma[J]. Cancer Metab, 2020, 8: 4.
doi: 10.1186/s40170-020-0209-8 pmid: 32158544 |
| [2] | FARES J, FARES M Y, KHACHFE H H, et al. Molecular principles of metastasis: a hallmark of cancer revisited[J]. Signal Transduct Target Ther, 2020, 5(1): 28. |
| [3] |
BERGERS G, FENDT S M. The metabolism of cancer cells during metastasis[J]. Nat Rev Cancer, 2021, 21(3): 162-180.
doi: 10.1038/s41568-020-00320-2 pmid: 33462499 |
| [4] |
LEONE R D, ZHAO L, ENGLERT J M, et al. Glutamine blockade induces divergent metabolic programs to overcome tumor immune evasion[J]. Science, 2019, 366(6468): 1013-1021.
doi: 10.1126/science.aav2588 pmid: 31699883 |
| [5] |
WANG Y Y, BAI C S, RUAN Y X, et al. Coordinative metabolism of glutamine carbon and nitrogen in proliferating cancer cells under hypoxia[J]. Nat Commun, 2019, 10(1): 201.
doi: 10.1038/s41467-018-08033-9 pmid: 30643150 |
| [6] |
KODAMA M, OSHIKAWA K, SHIMIZU H, et al. A shift in glutamine nitrogen metabolism contributes to the malignant progression of cancer[J]. Nat Commun, 2020, 11(1): 1320.
doi: 10.1038/s41467-020-15136-9 pmid: 32184390 |
| [7] |
ADHIKARY G, SHRESTHA S, NASELSKY W, et al. Mesothelioma cancer cells are glutamine addicted and glutamine restriction reduces YAP1 signaling to attenuate tumor formation[J]. Mol Carcinog, 2023, 62(4): 438-449.
doi: 10.1002/mc.v62.4 |
| [8] |
JIANG B, ZHANG J, ZHAO G H, et al. Filamentous GLS1 promotes ROS-induced apoptosis upon glutamine deprivation via insufficient asparagine synthesis[J]. Mol Cell, 2022, 82(10): 1821-1835.e6.
doi: 10.1016/j.molcel.2022.03.016 pmid: 35381197 |
| [9] |
VILLAR V H, ALLEGA M F, DESHMUKH R, et al. Hepatic glutamine synthetase controls N5-methylglutamine in homeostasis and cancer[J]. Nat Chem Biol, 2023, 19(3): 292-300.
doi: 10.1038/s41589-022-01154-9 |
| [10] |
SHANG M, CAPPELLESSO F, AMORIM R, et al. Macrophage-derived glutamine boosts satellite cells and muscle regeneration[J]. Nature, 2020, 587(7835): 626-631.
doi: 10.1038/s41586-020-2857-9 |
| [11] |
DORAI T, PINTO J T, DENTON T T, et al. The metabolic importance of the glutaminase Ⅱ pathway in normal and cancerous cells[J]. Anal Biochem, 2022, 644: 114083.
doi: 10.1016/j.ab.2020.114083 |
| [12] |
YOO H C, PARK S J, NAM M, et al. A variant of SLC1A5 is a mitochondrial glutamine transporter for metabolic reprogramming in cancer cells[J]. Cell Metab, 2020, 31(2): 267-283.e12.
doi: S1550-4131(19)30664-3 pmid: 31866442 |
| [13] |
GUO C S, YOU Z Y, SHI H, et al. SLC38A2 and glutamine signalling in cDC1s dictate anti-tumour immunity[J]. Nature, 2023, 620(7972): 200-208.
doi: 10.1038/s41586-023-06299-8 |
| [14] | LIU R, HONG R X, WANG Y, et al. Defect of SLC38A3 promotes epithelial-mesenchymal transition and predicts poor prognosis in esophageal squamous cell carcinoma[J]. Chung Kuo Yen Cheng Yen Chiu, 2020, 32(5): 547-563. |
| [15] |
CHEN Y Y, TAN L, GAO J, et al. Targeting glutaminase 1 (GLS1) by small molecules for anticancer therapeutics[J]. Eur J Med Chem, 2023, 252: 115306.
doi: 10.1016/j.ejmech.2023.115306 |
| [16] |
HAN T Y, WANG P C, WANG Y N, et al. FAIM regulates autophagy through glutaminolysis in lung adenocarcinoma[J]. Autophagy, 2022, 18(6): 1416-1432.
doi: 10.1080/15548627.2021.1987672 |
| [17] |
YU Y, YU X H, FAN C L, et al. Targeting glutaminase-mediated glutamine dependence in papillary thyroid cancer[J]. J Mol Med, 2018, 96(8): 777-790.
doi: 10.1007/s00109-018-1659-0 |
| [18] |
LIU H Y, ZHANG H S, LIU M Y, et al. GLS1 depletion inhibited colorectal cancer proliferation and migration via redox/Nrf2/autophagy-dependent pathway[J]. Arch Biochem Biophys, 2021, 708: 108964.
doi: 10.1016/j.abb.2021.108964 |
| [19] |
CAI J, CHEN Z Q, WANG J G, et al. circHECTD1 facilitates glutaminolysis to promote gastric cancer progression by targeting miR-1256 and activating β-catenin/c-Myc signaling[J]. Cell Death Dis, 2019, 10(8): 576.
doi: 10.1038/s41419-019-1814-8 pmid: 31371702 |
| [20] |
PASTUSHENKO I, BLANPAIN C. EMT transition states during tumor progression and metastasis[J]. Trends Cell Biol, 2019, 29(3): 212-226.
doi: S0962-8924(18)30201-0 pmid: 30594349 |
| [21] |
LI B H, CAO Y J, MENG G, et al. Targeting glutaminase 1 attenuates stemness properties in hepatocellular carcinoma by increasing reactive oxygen species and suppressing Wnt/beta-catenin pathway[J]. EBioMedicine, 2019, 39: 239-254.
doi: S2352-3964(18)30567-X pmid: 30555042 |
| [22] |
LUKEY M J, CLUNTUN A A, KATT W P, et al. Liver-type glutaminase GLS2 is a druggable metabolic node in luminal-subtype breast cancer[J]. Cell Rep, 2019, 29(1): 76-88.e7.
doi: S2211-1247(19)31131-3 pmid: 31577957 |
| [23] | SUZUKI S, VENKATESH D, KANDA H, et al. GLS2 is a tumor suppressor and a regulator of ferroptosis in hepatocellular carcinoma[J]. Cancer Res, 2022, 82(18): 3209-3222. |
| [24] |
ZHANG C, LIU J, ZHAO Y H, et al. Glutaminase 2 is a novel negative regulator of small GTPase Rac1 and mediates p53 function in suppressing metastasis[J]. Elife, 2016, 5: e10727.
doi: 10.7554/eLife.10727 |
| [25] |
KUO T C, CHEN C K, HUA K T, et al. Glutaminase 2 stabilizes dicer to repress snail and metastasis in hepatocellular carcinoma cells[J]. Cancer Lett, 2016, 383(2): 282-294.
doi: 10.1016/j.canlet.2016.10.012 |
| [26] |
DIAS M M, ADAMOSKI D, DOS REIS L M, et al. GLS2 is protumorigenic in breast cancers[J]. Oncogene, 2020, 39(3): 690-702.
doi: 10.1038/s41388-019-1007-z pmid: 31541193 |
| [27] |
BRABLETZ S, SCHUHWERK H, BRABLETZ T, et al. Dynamic EMT: a multi-tool for tumor progression[J]. EMBO J, 2021, 40(18): e108647.
doi: 10.15252/embj.2021108647 |
| [28] |
NALLASAMY P, NIMMAKAYALA R K, KARMAKAR S, et al. Pancreatic tumor microenvironment factor promotes cancer stemness via SPP1-CD44 axis[J]. Gastroenterology, 2021, 161(6): 1998-2013.e7.
doi: 10.1053/j.gastro.2021.08.023 pmid: 34418441 |
| [29] |
XIE W, JIANG Q W, WU X J, et al. IKBKE phosphorylates and stabilizes Snail to promote breast cancer invasion and metastasis[J]. Cell Death Differ, 2022, 29(8): 1528-1540.
doi: 10.1038/s41418-022-00940-1 pmid: 35066576 |
| [30] |
LEE M Y, LIM S, KIM Y S, et al. DEP-induced ZEB2 promotes nasal polyp formation via epithelial-to-mesenchymal transition[J]. J Allergy Clin Immunol, 2022, 149(1): 340-357.
doi: 10.1016/j.jaci.2021.04.024 |
| [31] |
LIANG Y P, CEN J J, HUANG Y, et al. CircNTNG1 inhibits renal cell carcinoma progression via HOXA5-mediated epigenetic silencing of Slug[J]. Mol Cancer, 2022, 21(1): 224.
doi: 10.1186/s12943-022-01694-7 pmid: 36536414 |
| [32] |
ANG H L, MOHAN C D, SHANMUGAM M K, et al. Mechanism of epithelial-mesenchymal transition in cancer and its regulation by natural compounds[J]. Med Res Rev, 2023, 43(4): 1141-1200.
doi: 10.1002/med.v43.4 |
| [33] |
RECOUVREUX M V, MOLDENHAUER M R, GALENKAMP K M O, et al. Glutamine depletion regulates Slug to promote EMT and metastasis in pancreatic cancer[J]. J Exp Med, 2020, 217(9): e20200388.
doi: 10.1084/jem.20200388 |
| [34] |
CHEN X T, HUANG L L, YANG T T, et al. METTL3 promotes esophageal squamous cell carcinoma metastasis through enhancing GLS2 expression[J]. Front Oncol, 2021, 11: 667451.
doi: 10.3389/fonc.2021.667451 |
| [35] |
KIM G W, LEE D H, JEON Y H, et al. Glutamine synthetase as a therapeutic target for cancer treatment[J]. Int J Mol Sci, 2021, 22(4): 1701.
doi: 10.3390/ijms22041701 |
| [36] |
ZHANG R, ZHU J C, HU H, et al. MicroRNA-140-5p suppresses invasion and proliferation of glioma cells by targeting glutamate-ammonia ligase (GLUL)[J]. Neoplasma, 2020, 67(2): 371-378.
doi: 10.4149/neo_2020_190514N432 pmid: 31986891 |
| [37] |
MENGA A, FAVIA M, SPERA I, et al. N-acetylaspartate release by glutaminolytic ovarian cancer cells sustains protumoral macrophages[J]. EMBO Rep, 2021, 22(9): e51981.
doi: 10.15252/embr.202051981 |
| [38] |
XUAN D T M, WU C C, WANG W J, et al. Glutamine synthetase regulates the immune microenvironment and cancer development through the inflammatory pathway[J]. Int J Med Sci, 2023, 20(1): 35-49.
doi: 10.7150/ijms.75625 pmid: 36619229 |
| [39] |
QUAIL D F, JOYCE J A. Microenvironmental regulation of tumor progression and metastasis[J]. Nat Med, 2013, 19(11): 1423-1437.
doi: 10.1038/nm.3394 pmid: 24202395 |
| [40] |
LIN Y X, XU J X, LAN H Y. Tumor-associated macrophages in tumor metastasis: Biological roles and clinical therapeutic applications[J]. J Hematol Oncol, 2019, 12(1): 76.
doi: 10.1186/s13045-019-0760-3 |
| [41] |
WANG Y, GAO R F, LI J P, et al. Downregulation of hsa_circ_0074854 suppresses the migration and invasion in hepatocellular carcinoma via interacting with HuR and via suppressing exosomes-mediated macrophage M2 polarization[J]. Int J Nanomedicine, 2021, 16: 2803-2818.
doi: 10.2147/IJN.S284560 |
| [42] |
WEI C, YANG C G, WANG S Y, et al. Crosstalk between cancer cells and tumor associated macrophages is required for mesenchymal circulating tumor cell-mediated colorectal cancer metastasis[J]. Mol Cancer, 2019, 18(1): 64.
doi: 10.1186/s12943-019-0976-4 pmid: 30927925 |
| [43] |
PALMIERI E M, MENGA A, MARTÍN-PÉREZ R, et al. Pharmacologic or genetic targeting of glutamine synthetase skews macrophages toward an M1-like phenotype and inhibits tumor metastasis[J]. Cell Rep, 2017, 20(7): 1654-1666.
doi: S2211-1247(17)31033-1 pmid: 28813676 |
| [44] |
MENGA A, SERRA M, TODISCO S, et al. Glufosinate constrains synchronous and metachronous metastasis by promoting anti-tumor macrophages[J]. EMBO Mol Med, 2020, 12(10): e11210.
doi: 10.15252/emmm.201911210 |
| [45] |
WANG T, CAI B L, DING M C, et al. C-myc overexpression promotes oral cancer cell proliferation and migration by enhancing glutaminase and glutamine synthetase activity[J]. Am J Med Sci, 2019, 358(3): 235-242.
doi: S0002-9629(19)30219-8 pmid: 31324362 |
| [46] |
BODINEAU C, TOMÉ M, MURDOCH P D S, et al. Glutamine, MTOR and autophagy: a multiconnection relationship[J]. Autophagy, 2022, 18(11): 2749-2750.
doi: 10.1080/15548627.2022.2062875 |
| [47] |
JIN L T, CHUN J, PAN C Y, et al. The PLAG1-GDH1 axis promotes anoikis resistance and tumor metastasis through CamKK2-AMPK signaling in LKB1-deficient lung cancer[J]. Mol Cell, 2018, 69(1): 87-99.e7.
doi: S1097-2765(17)30884-5 pmid: 29249655 |
| [48] |
KANG J, CHUN J, HWANG J S, et al. EGFR-phosphorylated GDH1 harmonizes with RSK2 to drive CREB activation and tumor metastasis in EGFR-activated lung cancer[J]. Cell Rep, 2022, 41(11): 111827.
doi: 10.1016/j.celrep.2022.111827 |
| [49] |
LIU G J, ZHU J, YU M L, et al. Glutamate dehydrogenase is a novel prognostic marker and predicts metastases in colorectal cancer patients[J]. J Transl Med, 2015, 13: 144.
doi: 10.1186/s12967-015-0500-6 pmid: 25947346 |
| [50] | MAO L, HONG X, XU L W, et al. Sirtuin 4 inhibits prostate cancer progression and metastasis by modulating p21 nuclear translocation and glutamate dehydrogenase 1 ADP-ribosylation[J]. J Oncol, 2022, 2022: 5498743. |
| [51] |
LEE H T, HUANG C H, CHEN W C, et al. Transglutaminase 2 promotes migration and invasion of lung cancer cells[J]. Oncol Res, 2018, 26(8): 1175-1182.
doi: 10.3727/096504018X15149761920868 |
| [52] |
ECKERT R L. Transglutaminase 2 takes center stage as a cancer cell survival factor and therapy target[J]. Mol Carcinog, 2019, 58(6): 837-853.
doi: 10.1002/mc.v58.6 |
| [53] |
KANG S, OH S C, MIN B W, et al. Transglutaminase 2 regulates self-renewal and stem cell marker of human colorectal cancer stem cells[J]. Anticancer Res, 2018, 38(2): 787-794.
pmid: 29374703 |
| [54] |
JIA C C, WANG G Y, WANG T T, et al. Cancer-associated fibroblasts induce epithelial-mesenchymal transition via the transglutaminase 2-dependent IL-6/IL6R/STAT3 axis in hepatocellular carcinoma[J]. Int J Biol Sci, 2020, 16(14): 2542-2558.
doi: 10.7150/ijbs.45446 |
| [55] |
ASHOUR A A, GURBUZ N, ALPAY S N, et al. Elongation factor-2 kinase regulates TG2/β1 integrin/Src/uPAR pathway and epithelial-mesenchymal transition mediating pancreatic cancer cells invasion[J]. J Cell Mol Med, 2014, 18(11): 2235-2251.
doi: 10.1111/jcmm.12361 pmid: 25215932 |
| [56] |
WANG X F, YU Z J, ZHOU Q, et al. Tissue transglutaminase-2 promotes gastric cancer progression via the ERK1/2 pathway[J]. Oncotarget, 2016, 7(6): 7066-7079.
doi: 10.18632/oncotarget.6883 pmid: 26771235 |
| [57] |
DING Y, LIU P F, ZHANG S S, et al. Screening pathogenic genes in oral squamous cell carcinoma based on the mRNA expression microarray data[J]. Int J Mol Med, 2018, 41(6): 3597-3603.
doi: 10.3892/ijmm.2018.3514 pmid: 29512771 |
| [58] |
CSANADI A, OSER A, AUMANN K, et al. Overexpression of SLC1a5 in lymph node metastases outperforms assessment in the primary as a negative prognosticator in non-small cell lung cancer[J]. Pathology, 2018, 50(3): 269-275.
doi: 10.1016/j.pathol.2017.10.016 |
| [59] |
DING M C, BU X, LI Z H, et al. NDRG2 ablation reprograms metastatic cancer cells towards glutamine dependence via the induction of ASCT2[J]. Int J Biol Sci, 2020, 16(16): 3100-3115.
doi: 10.7150/ijbs.48066 |
| [60] |
WANG Y H, FU L, CUI M Q, et al. Amino acid transporter SLC38A3 promotes metastasis of non-small cell lung cancer cells by activating PDK1[J]. Cancer Lett, 2017, 393: 8-15.
doi: S0304-3835(17)30078-2 pmid: 28202352 |
| [61] |
ZHANG D J, ZHAO L, SHEN Q, et al. Down-regulation of KIAA1199/CEMIP by miR-216a suppresses tumor invasion and metastasis in colorectal cancer[J]. Int J Cancer, 2017, 140(10): 2298-2309.
doi: 10.1002/ijc.30656 pmid: 28213952 |
| [62] |
MOROTTI M, ZOIS C E, EL-ANSARI R, et al. Increased expression of glutamine transporter SNAT2/SLC38A2 promotes glutamine dependence and oxidative stress resistance, and is associated with worse prognosis in triple-negative breast cancer[J]. Br J Cancer, 2021, 124(2): 494-505.
doi: 10.1038/s41416-020-01113-y |
| [63] |
NAJUMUDEEN A K, CETECI F, FEY S K, et al. The amino acid transporter SLC7A5 is required for efficient growth of KRAS-mutant colorectal cancer[J]. Nat Genet, 2021, 53(1): 16-26.
doi: 10.1038/s41588-020-00753-3 pmid: 33414552 |
| [64] |
INNAO V, RIZZO V, ALLEGRA A G, et al. Promising anti-mitochondrial agents for overcoming acquired drug resistance in multiple myeloma[J]. Cells, 2021, 10(2): 439.
doi: 10.3390/cells10020439 |
| [65] |
RICHARD S M, MARTINEZ MARIGNAC V L. Sensitization to oxaliplatin in HCT116 and HT29 cell lines by metformin and ribavirin and differences in response to mitochondrial glutaminase inhibition[J]. J Cancer Res Ther, 2015, 11(2): 336-340.
doi: 10.4103/0973-1482.157317 pmid: 26148596 |
| [66] |
HALAMA A, KULINSKI M, DIB S S, et al. Accelerated lipid catabolism and autophagy are cancer survival mechanisms under inhibited glutaminolysis[J]. Cancer Lett, 2018, 430: 133-147.
doi: S0304-3835(18)30342-2 pmid: 29777783 |
| [1] | FENG Xinying, WANG Bing, LIU Peifeng. Innovations and challenges in intraperitoneal chemotherapy for peritoneal metastatic carcinoma [J]. China Oncology, 2024, 34(9): 827-837. |
| [2] | CAO Xiaoshan, YANG Beibei, CONG Binbin, LIU Hong. The progress of treatment for brain metastases of triple-negative breast cancer [J]. China Oncology, 2024, 34(8): 777-784. |
| [3] | HUANG Sijie, KANG Xun, LI Wenbin. Clinical research progress of intrathecal therapy in the treatment of leptomeningeal metastasis [J]. China Oncology, 2024, 34(7): 695-701. |
| [4] | SUN Rongqi, SONG Ning, ZHENG Wentian, ZHANG Xinyue, LI Minmin, GONG Hui, JIANG Yingying. Effect of long noncoding RNA FLJ30679 on proliferation and migration of oral squamous cell carcinoma cells [J]. China Oncology, 2024, 34(5): 439-450. |
| [5] | XU Yonghu, XU Dazhi. Progress and prospects of gastric cancer treatment in the 21st century [J]. China Oncology, 2024, 34(3): 239-249. |
| [6] | CHEN Yifan, LI Ting, WANG Biyun. Research progress of CCR8 in tumor immunotherapy [J]. China Oncology, 2024, 34(3): 299-305. |
| [7] | JIN Yizi, LIN Mingxi, ZENG Cheng, GUO Qing, ZHANG Jian. Research advances in estrogen receptor low positive early breast cancer [J]. China Oncology, 2024, 34(10): 972-978. |
| [8] | JIA Liqing, GE Xiaolu, JIANG Lin, ZHOU Xiaoyan. Effects of lncRNA PKD2-2-3 on cell proliferation, clone formation, migration, and invasion of lung adenocarcinoma [J]. China Oncology, 2023, 33(8): 717-725. |
| [9] | KANG Yinnan, CHEN Shun, XIE Youcheng, ZHENG Ying, HE Yujing, LI Chuyi, YU Xiaohui. Application and research progress of antibody drug conjugates in HER2 positive advanced gastric cancer [J]. China Oncology, 2023, 33(8): 790-800. |
| [10] | WU Jing, ZHOU Juan, SU Chunxia. Advances in fatty acid metabolism reprogramming of lung cancer [J]. China Oncology, 2023, 33(5): 517-526. |
| [11] | JIANG Jinling, ZHOU Chenfei, WANG Chao, ZHAO Liqin, WU Junwei, ZHANG Jun. Advanced progress in research and diagnosis of gastric cancer in 2022 [J]. China Oncology, 2023, 33(4): 303-314. |
| [12] | TIAN Xi, XU Wenhao, ZHU Shuxuan, AIHETAIMUJIANG•Anwaier, SU Jiaqi, YE Shiqi, QU Yuanyuan, SHI Guohai, ZHANG Hailiang, YE Dingwei. Advances in the research, diagnosis and treatment of renal cell carcinoma in 2022 [J]. China Oncology, 2023, 33(3): 191-200. |
| [13] | SU Chunxia, ZHOU Caicun. Important clinical research progress in lung cancer in 2022 [J]. China Oncology, 2023, 33(3): 218-227. |
| [14] | CAO Xiaoshan, CONG Binbin. The research progress of endocrine therapy combined with targeted therapy for triple-positive breast cancer [J]. China Oncology, 2023, 33(3): 288-292. |
| [15] | SHAO Zhibo, YANG Benlong, WU Jiong. Progress of important clinical trials of breast cancer in China in 2022 [J]. China Oncology, 2023, 33(2): 103-109. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
沪ICP备12009617
Powered by Beijing Magtech Co. Ltd
