Welcome to China Oncology,
China Oncology ›› 2024, Vol. 34 ›› Issue (9): 827-837.doi: 10.19401/j.cnki.1007-3639.2024.09.003
• Specialists' Commentory • Previous Articles Next Articles
FENG Xinying1( ), WANG Bing1, LIU Peifeng1,2(
), WANG Bing1, LIU Peifeng1,2( )
)
Received:2024-08-01
Revised:2024-09-11
Online:2024-09-30
Published:2024-10-11
Contact:
LIU Peifeng
Share article
CLC Number:
FENG Xinying, WANG Bing, LIU Peifeng. Innovations and challenges in intraperitoneal chemotherapy for peritoneal metastatic carcinoma[J]. China Oncology, 2024, 34(9): 827-837.
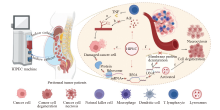
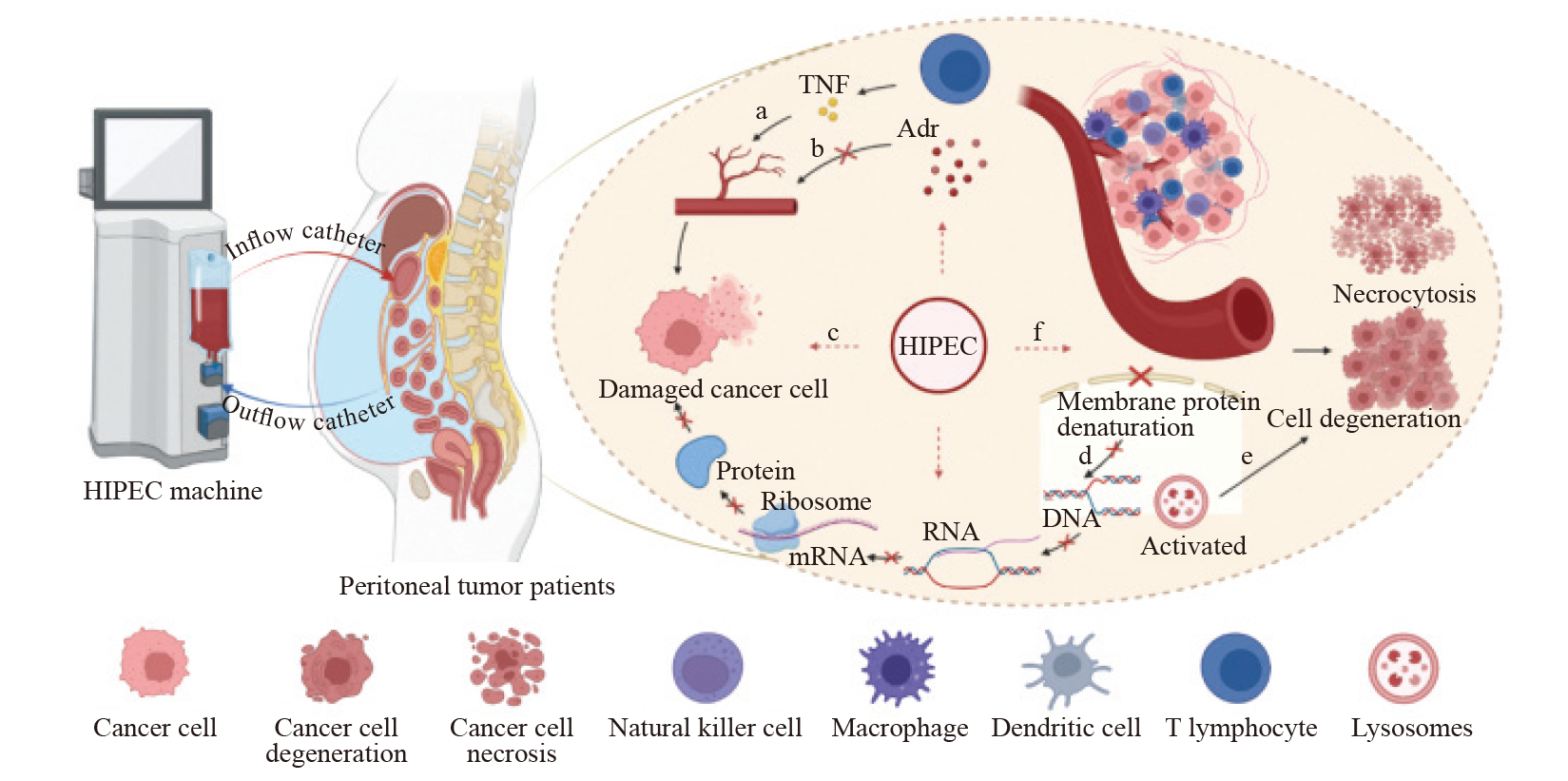
Fig. 2
The warm effect of HIPEC a: TNF selectively disrupts blood vessels within tumor tissue, leading to thrombosis and ischemic necrosis. b: Tumor tissue, when exposed to heat stimuli, cannot regulate vasodilation via Adr, resulting in increased blood flow that fails to achieve thermoregulation. c: Cancer cells in the S/M phase are sensitive to hyperthermia, which induces cell death. d: Hyperthermia causes denaturation of membrane proteins, disrupting the synthesis of DNA, RNA and proteins. e: Heat stress activates lysosomes, causing cytoplasmic and nuclear damage, ultimately leading to cell apoptosis. f: Hyperthermia can trigger the immune system by activating heat shock proteins, stimulating various immune cells, including natural killer cells, macrophages, dendritic cells and T lymphocytes. This process inhibits angiogenesis, causing hypoxia, acidosis or nutrient deprivation, leading to cellular degeneration and necrosis. TNF: Tumor necrosis factor; Adr: Adrenaline. Created with BioRender.com."
Tab. 1
Commonly used IPC protocols in clinical practice"
| Preoperative diagnosis | Surgical program | Research type | Number of sample | Chemotherapy protocol | Result | Reference | |||
|---|---|---|---|---|---|---|---|---|---|
| Trial | Control | Trial | Control | ||||||
| Gastric cancer +Peritoneal cancer | CRS | Gastripec-Ⅰ | 52 | 53 | CRS+HIPEC (mitomycin 15 mg/m2+cisplatin 75 mg/m2, 42 ℃, 60 min) | Simple CRS; 11 cases of postoperative chemotherapy (such as fluorouracil) | Better PFS and MFS | [ | |
| Gastric cancer (c>T3) | Radical gastrectomy for gastric cancer+D2 lymphnode dissection | Phase Ⅲ clinical trial | 40 | 40 | CRS+HIPEC [cisplatin 50 mg/m2, 60 min, (42.0±1.0) ℃]; 22 cases within 1 month after surgery XELOX regimen with 6 standard doses | XELOX regimen with 6 standard doses within 1 month after surgery | Low DFS and peritoneal recurrence | [ | |
| Colon cancer (T4N0-2M0) | CRS | Phase Ⅲ clinical trial | 89 | 95 | CRS+HIPEC (mitomycin 30 mg/m2 ); Receive routine adjuvant systemic chemotherapy combined with oxaliplatin and capecitabine within 12 weeks after surgery | CRS+systemic chemotherapy combined with oxaliplatin and capecitabine within 12 weeks | Improve 3 years LC rate | [ | |
| Colon cancer (T4N0-2M0) | CRS | Phase Ⅲ clinical trial | 100 | 102 | HIPEC after surgery 5-8 weeks (oxaliplatin 460 mg/m2, 30 min)+ chemotherapy with intravenous fluorouracil/calcium folinate | Hydrostatic injection of fluorouracil/calcium folinate adjuvant chemotherapy | No significant difference | [ | |
| Appendix/colorectal cancer+peritoneal cancer | Phase Ⅰ clinical trial | 12 | PIPAC (oxaliplatin 90 mg/m2 )+whole body chemotherapy (fluorouracil+calcium folinate) | Safe and feasible | [ | ||||
| Phase Ⅲ/Ⅳ ovarian cancer | CRS | Phase Ⅲ clinical trial | 92 | 92 | CRS+HIPEC (cisplatin 75 mg/m2, 90 min, 41.5 ℃); 6 cycles of adjuvant chemotherapy (paclitaxel 175 mg/m2 + carboplatin 5 mg/mL) | 6 cycles of adjuvant chemotherapy (paclitaxel 175 mg/m2 +carboplatin 5 mg/mL) | Cannot improve PFS and OS | [ | |
| Ovarian cancer | CRS | Clinical research | 100 | PIPAC (cisplatin 7.5 mg/m2 + doxorubicin 1.5 mg/m2) | The result is meaningless | [ | |||
| Breast cancer/endometrial cancer+peritoneal cancer | Hysterectom/mastectomy | Cohort study | 44 | PIPAC (cisplatin 7.5 mg/m2 +doxorubicin 1.5 mg/m2); Later changed to PIPAC (cisplatin 10.5 mg/m2 +doxorubicin 2.1 mg/m2) | Improve survival rate and histological regression | [ | |||
| Mucinous carcinoma of the appendix +PMP | CRS+Peritoneal resection surgery | Case report | 1 | CRS+HIPEC (mitomycin 35 mg/m2, 42 ℃, 90 min) | Improve survival rate | [ | |||
| Appendiceal tumor +PMP | CRS+Peritoneal resection surgery | Case report | 1 | CRS+IPC (cisplatin 30 mg/m2+ docetaxel 30 mg/m2)+ HIPEC (oxaliplatin 4 300 mg+fluorouracil 500 mg, 42.5-43.5 ℃, 40 min) | Good postoperative recovery | [ | |||
| Intrahepatic cholangiocarcinoma + peritoneal cancer | CRS | Cohort study | 51 | 61 | CRS+HIPEC (fluorouracil 1 000 mg/BSA+cisplatin 40 mg/m2, 400-600 mL/min)+ IPC (fluorouracil or oxaliplatin) | Simple CRS +IPC (fluorouracil or oxaliplatin) | Improve OS and survival rate | [ | |
| Pancreatic cancer +peritoneal cancer | Pancreatic surgery or systemic chemotherapy | Prospective study | 35 | PIPAC (30 mL/min, cisplatin 7.5 mg/m2+ doxorubicin 1.5 mg/m2, 92 min) | Improve survival rate and histological regression | [ | |||
Tab. 2
Research on nanodrugs in IPC"
| Carrier | Material | Load | Cell/animal model | Administration method | Indication | Advantage/result | Reference |
|---|---|---|---|---|---|---|---|
| Polymeric micelles | Polyethylene glycol, lactic acid hydroxy acetic acid copolymer | Oxaliplatin | Tumor bearing mouse model | IP, IV | Colorectal cancer with peritoneal metastasis | IP reduces the systemic distribution of drugs and the toxicity to major organs, improves the precision of treatment | [ |
| Nano hydrogel | Carboxymethyl chitosan, polylactic acid hyperbranched polyglycerol | Paclitaxel, anti-PD-1 antibody | C57BL/6 mouse model | IP | Ovarian cancer with peritoneal metastasis | ① Low system toxicity and good therapeutic effect; ② Good biocompatibility | [ |
| 14C-eNP | eNPs, 14C | Paclitaxel | Mesothelioma of peritoneum tumor bearing mouse model | IP, IV | Mesothelioma of peritoneum | IP efficiency is higher | [ |
| Liposomes | Liposomes encoding firefly luciferase mRNA | mRNA | C57BL/6 mouse model | IP, IV | Pancreatic related cancer | IP enhances the ability of mRNA delivery to the pancreas | [ |
| Liposomes | Cationic assisted lipids, liposomes encoding firefly luciferase mRNA | mRNA | C57BL/6 mouse model | IP, IV | Pancreatic related cancer | IP reduces off target delivery to the liver and spleen | [ |
| Liposomes | Phosphatidylserine, β-sitosterol | mRNA | CT26-luc mouse model | IP | High transfection efficiency and selectivity | [ | |
| CAR-Ms | PD-1 antibody | CT26 mouse model | IP | Late stage diffuse peritoneal tumor | Activate adaptive immune response provide new strategies for solid tumor treatment | [ | |
| Liposomes | C12-200 cation, Cy5.5 dye | siRNA | Mouse model | IP | Enhancing the potential of using LPM to transport oligonucleotides encapsulated in nanoparticles for cancer treatment | [ | |
| Liposomes | 4A3-SCC-10/PH | mRNA | tdTomato mouse model | IP | Liver cancer | Improving mRNA delivery efficiency in vivo has the potential to treat liver cancer | [ |
| Nanoparticles | Silicon dioxide, polystyrene nanoparticles, polylactic acid hydroxyacetic acid copolymer | Mouse model | IP, IV | Ovarian cancer with abdominal metastasis | ① IP has high targeting efficiency; ② Lay the foundation for the treatment of metastatic ovarian cancer | [ | |
| Nanoparticles | PEG5k-BA4, PEG5k-Cys4-L8-CA8 | Paclitaxel, Betulinic acid | A2780/Adr cells, ovarian cancer mouse model | IP | Ovarian cancer | ① Achieve precise drug release; ② Overcome multidrug resistance in ovarian cancer | [ |
| [1] | 中国抗癌协会腹膜肿瘤专业委员会. 中国肿瘤整合诊治指南:腹膜肿瘤(胃肠肿瘤部分)[J]. 消化肿瘤杂志(电子版), 2023, 15(2): 100-108. |
| Chinese Society of Peritoneal Oncology, China Anti-Cancer Association. China Anti-Cancer Association guidelines for holistic integrative management of cancer-peritoneal tumours from gastrointestinal tract[J]. J Digest Oncol (Elect Ver), 2023, 15(2): 100-108. | |
| [2] | 唐鸿生, 阮强, 崔书中. 《中国肿瘤整合诊治指南: 腹膜肿瘤》解读[J]. 消化肿瘤杂志(电子版), 2023, 15(2): 109-113. |
| TANG H S, RUAN Q, CUI S Z. Interpretation of China Anti-Cancer Association (CACA) guidelines for holistic integrative management of cancer-peritoneal tumours[J]. J Digest Oncol (Elect Ver), 2023, 15(2): 109-113. | |
| [3] | LEI Z Y, WANG J H, LI Z, et al. Hyperthermic intraperitoneal chemotherapy for gastric cancer with peritoneal metastasis: a multicenter propensity score-matched cohort study[J]. Chin J Cancer Res, 2020, 32(6): 794-803. |
| [4] | 关天培, 雷子颖, 崔书中. 结肠直肠癌腹膜转移防治临床研究[J]. 外科理论与实践, 2021, 26(1): 7-10. |
| GUAN T P, LEI Z Y, CUI S Z. Clinical study on prevention and treatment of peritoneal metastasis of colorectal cancer[J]. J Surg Concepts Pract, 2021, 26(1): 7-10. | |
| [5] | 樊代明, 崔书中. 中国肿瘤整合诊治指南:腹膜肿瘤(2022)[M]. 天津科学技术出版社, 2022. |
| FAN D M, CUI S Z. Guidelines for integrated diagnosis and treatment of tumors in china: peritoneal tumors (2022)[M]. Tianjin Sci Technol Press, 2022. | |
| [6] | HOSSAIN M B, HALDAR NEER A H. Chemotherapy[M]//QAZI A S, TARIQ K. Therapeutic approaches in cancer treatment: vol. 185. Berlin: Springer, 2023: 49-58. |
| [7] | JACQUET P, SUGARBAKER P H. Peritoneal-plasma barrier[M]//SUGARBAKER P H. Peritoneal carcinomatosis: principles of management. Berlin: Springer, 1996: 53-63. |
| [8] |
SADEGHI B, ARVIEUX C, GLEHEN O, et al. Peritoneal carcinomatosis from non-gynecologic malignancies: results of the EVOCAPE 1 multicentric prospective study[J]. Cancer, 2000, 88(2): 358-363.
doi: 10.1038/nrc.2016.108 pmid: 27834398 |
| [9] | JAABACK K, JOHNSON N, LAWRIE T A. Intraperitoneal chemotherapy for the initial management of primary epithelial ovarian cancer[J]. Cochrane Database Syst Rev, 2016, 2016(1): CD005340. |
| [10] | CARLIER C, MATHYS A, JAEGHERE E D, et al. Tumour tissue transport after intraperitoneal anticancer drug delivery[J]. Int J Hyperthermia, 2017, 33(5): 534-542. |
| [11] | GUCHELAAR N A D, NASSERINEJAD K, MOSTERT B, et al. Intraperitoneal chemotherapy for peritoneal metastases of gastric origin: a systematic review and meta-analysis[J]. Br J Surg, 2024, 111(5): znae116. |
| [12] | SHI J J, KANTOFF P W, WOOSTER R, et al. Cancer nanomedicine: progress, challenges and opportunities[J]. Nat Rev Cancer, 2017, 17(1): 20-37. |
| [13] | AL-QUTEIMAT O M, AL-BADAINEH M A. Intraperitoneal chemotherapy: rationale, applications, and limitations[J]. J Oncol Pharm Pract, 2014, 20(5): 369-380. |
| [14] |
FUJIMOTO S, SHRESTHA R D, KOKUBUN M, et al. Intraperitoneal hyperthermic perfusion combined with surgery effective for gastric cancer patients with peritoneal seeding[J]. Ann Surg, 1988, 208(1):36-41.
doi: 10.1097/00000658-198807000-00005 pmid: 3133994 |
| [15] | TEMPFER C B, SOLASS W, REYMOND M A. Pressurized intraperitoneal chemotherapy (PIPAC) in women with gynecologic malignancies: a review[J]. Wien Med Wochenschr, 2014, 164(23/24): 519-528. |
| [16] |
SOLASS W, KERB R, MÜRDTER T, et al. Intraperitoneal chemotherapy of peritoneal carcinomatosis using pressurized aerosol as an alternative to liquid solution: first evidence for efficacy[J]. Ann Surg Oncol, 2014, 21(2): 553-559.
doi: 10.1245/s10434-013-3213-1 pmid: 24006094 |
| [17] |
COCCOLINI F, COTTE E, GLEHEN O, et al. Intraperitoneal chemotherapy in advanced gastric cancer. Meta-analysis of randomized trials[J]. Eur J Surg Oncol, 2014, 40(1): 12-26.
doi: 10.1016/j.ejso.2013.10.019 pmid: 24290371 |
| [18] | FILIS P, MAURI D, MARKOZANNES G, et al. Hyperthermic intraperitoneal chemotherapy (HIPEC) for the management of primary advanced and recurrent ovarian cancer: a systematic review and meta-analysis of randomized trials[J]. ESMO Open, 2022, 7(5): 100586. |
| [19] | CORTÉS-GUIRAL D, HÜBNER M, ALYAMI M, et al. Primary and metastatic peritoneal surface malignancies[J]. Nat Rev Dis Primers, 2021, 7(1): 91. |
| [20] | SANTULLO F, PACELLI F, ABATINI C, et al. Cytoreduction and hyperthermic intraperitoneal chemotherapy for pseudomyxoma peritonei of appendiceal origin: a single center experience[J]. Front Surg, 2021, 8: 715119. |
| [21] | KUSAMURA S, BARRETTA F, YONEMURA Y, et al. The role of hyperthermic intraperitoneal chemotherapy in pseudomyxoma peritonei after cytoreductive surgery[J]. JAMA Surg, 2021, 156(3): e206363. |
| [22] | PAPANTONI E, NTATSIS K, KYZIRIDIS D, et al. Twenty-years’ experience with cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) for pseudomyxoma peritonei (PMP)[J]. J BUON, 2021, 26(4): 1647-1652. |
| [23] |
MAJEED A, ALAPARTHI S, HALEGOUA-DEMARZIO D, et al. Complete pathologic response to gemcitabine and oxaliplatin chemotherapy after prior therapies in a patient with hepatocellular carcinoma and peritoneal metastases undergoing cytoreductive surgery and hyperthermic intraperitoneal chemotherapy[J]. World J Oncol, 2024, 15(3): 511-520.
doi: 10.14740/wjon1840 pmid: 38751709 |
| [24] | HUNG K C, YANG K L, HUANG G C, et al. Cytoreduction surgery and hyperthermic intraperitoneal chemotherapy for treating advanced peritoneal metastases of hepatocellular carcinoma[J]. Pleura Peritoneum, 2020, 5(2): 20190030. |
| [25] | HERNANDEZ D L, RESTREPO J, GARCIA MORA M. Peritoneal metastasis of cholangiocarcinoma treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy at the instituto nacional de cancerología, Colombia[J]. Cureus, 2020, 12(1): e6697. |
| [26] |
FRASSINI S, CALABRETTO F, GRANIERI S, et al. Intraperitoneal chemotherapy in the management of pancreatic adenocarcinoma: a systematic review and meta-analysis[J]. Eur J Surg Oncol, 2022, 48(9): 1911-1921.
doi: 10.1016/j.ejso.2022.05.030 pmid: 35688711 |
| [27] | RAU B, LANG H, KOENIGSRAINER A, et al. Effect of hyperthermic intraperitoneal chemotherapy on cytoreductive surgery in gastric cancer with synchronous peritoneal metastases: the phase Ⅲ GASTRIPEC-I trial[J]. J Clin Oncol, 2024, 42(2): 146-156. |
| [28] | LIU G, JI Z H, YU Y, et al. Treatment of hypermyoglobinemia after CRS+HIPEC for patients with peritoneal carcinomatosis: a retrospective comparative study[J]. Medicine, 2017, 96(45): e8573. |
| [29] | BRANDL A, ZIELINSKI C B, RAUE W, et al. Peritoneal metastases of rare carcinomas treated with cytoreductive surgery and HIPEC-a single center case series[J]. Ann Med Surg, 2017, 22: 7-11. |
| [30] |
GLEHEN O, MOHAMED F, GILLY F N. Peritoneal carcinomatosis from digestive tract cancer: new management by cytoreductive surgery and intraperitoneal chemohyperthermia[J]. Lancet Oncol, 2004, 5(4): 219-228.
doi: 10.1016/S1470-2045(04)01425-1 pmid: 15050953 |
| [31] |
FRIEDRICH M, ZINN W, KOLNSBERG L, et al. Hyperthermic intraperitoneal chemotherapy (HIPEC) for ovarian cancer: evaluation of side effects in a single institution cohort[J]. Anticancer Res, 2020, 40(3): 1481-1486.
doi: 10.21873/anticanres.14092 pmid: 32132047 |
| [32] | ALYAMI M, HÜBNER M, GRASS F, et al. Pressurised intraperitoneal aerosol chemotherapy: rationale, evidence, and potential indications[J]. Lancet Oncol, 2019, 20(7): e368-e377. |
| [33] | GUEL-KLEIN S, ALBERTO VILCHEZ M E, CEELEN W, et al. Is PIPAC a treatment option in upper and lower gastrointestinal cancer with peritoneal metastasis?[J]. Visc Med, 2022, 38(2): 90-98. |
| [34] |
COLBY A H, KIRSCH J, PATWA A N, et al. Radiolabeled biodistribution of expansile nanoparticles: intraperitoneal administration results in tumor specific accumulation[J]. ACS Nano, 2023, 17(3): 2212-2221.
doi: 10.1021/acsnano.2c08451 pmid: 36701244 |
| [35] |
MOUKARZEL L A, FERRANDO L, DOPESO H, et al. Hyperthermic intraperitoneal chemotherapy (HIPEC) with carboplatin induces distinct transcriptomic changes in ovarian tumor and normal tissues[J]. Gynecol Oncol, 2022, 165(2): 239-247.
doi: 10.1016/j.ygyno.2022.02.022 pmid: 35292180 |
| [36] | RAOOF M, WHELAN R L, SULLIVAN K M, et al. Safety and efficacy of oxaliplatin pressurized intraperitoneal aerosolized chemotherapy (PIPAC) in colorectal and appendiceal cancer with peritoneal metastases: results of a multicenter phase Ⅰ trial in the USA[J]. Ann Surg Oncol, 2023, 30(12): 7814-7824. |
| [37] | ZHANG Y M, WANG S L, DUAN X F, et al. mPEG-PDLLA micelles potentiate docetaxel for intraperitoneal chemotherapy in ovarian cancer peritoneal metastasis[J]. Front Pharmacol, 2022, 13: 861938. |
| [38] | SHIMIZU T, SONODA H, MURATA S, et al. Hyperthermic intraperitoneal chemotherapy using a combination of mitomycin C, 5-fluorouracil, and oxaliplatin in patients at high risk of colorectal peritoneal metastasis: a phase Ⅰ clinical study[J]. Eur J Surg Oncol, 2014, 40(5): 521-528. |
| [39] | SUGARBAKER P H. Optimizing regional chemotherapy for epithelial ovarian cancer[J]. J Obstet Gynaecol Res, 2022, 48(6): 1306-1317. |
| [40] |
NADIRADZE G, GIGER-PABST U, ZIEREN J, et al. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) with low-dose cisplatin and doxorubicin in gastric peritoneal metastasis[J]. J Gastrointest Surg, 2016, 20(2): 367-373.
doi: 10.1007/s11605-015-2995-9 pmid: 26511950 |
| [41] | HELDERMAN R F C P A, LÖKE D R, VERHOEFF J, et al. The temperature-dependent effectiveness of platinum-based drugs mitomycin-C and 5-FU during hyperthermic intraperitoneal chemotherapy (HIPEC) in colorectal cancer cell lines[J]. Cells, 2020, 9(8): 1775. |
| [42] |
BEEHARRY M K, ZHU Z L, LIU W T, et al. Prophylactic HIPEC with radical D2 gastrectomy improves survival and peritoneal recurrence rates for locally advanced gastric cancer: Personal experience from a randomized case control study[J]. BMC Cancer, 2019, 19(1): 932.
doi: 10.1186/s12885-019-6125-z pmid: 31533660 |
| [43] | ARJONA-SÁNCHEZ A, ESPINOSA-REDONDO E, GUTIÉRREZ-CALVO A, et al. Efficacy and safety of intraoperative hyperthermic intraperitoneal chemotherapy for locally advanced colon cancer: a phase 3 randomized clinical trial[J]. JAMA Surg, 2023, 158(7): 683-691. |
| [44] | ZWANENBURG E S, KLAVER C E, WISSELINK D D, et al. Adjuvant hyperthermic intraperitoneal chemotherapy in patients with locally advanced colon cancer (COLOPEC): 5-year results of a randomized multicenter trial[J]. J Clin Oncol, 2024, 42(2): 140-145. |
| [45] | LIM M C, CHANG S J, PARK B, et al. Survival after hyperthermic intraperitoneal chemotherapy and primary or interval cytoreductive surgery in ovarian cancer: a randomized clinical trial[J]. JAMA Surg, 2022, 157(5): 374-383. |
| [46] | TALIENTO C, RESTAINO S, SCUTIERO G, et al. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) with cisplatin and doxorubicin in patients with ovarian cancer: a systematic review[J]. Eur J Surg Oncol, 2023, 49(12): 107250. |
| [47] |
REZNICZEK G A, GIGER-PABST U, THAHER O, et al. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) for rare gynecologic indications: peritoneal metastases from breast and endometrial cancer[J]. BMC Cancer, 2020, 20(1): 1122.
doi: 10.1186/s12885-020-07627-1 pmid: 33213407 |
| [48] | AWAD A, AWAD M, ALAMI M, et al. Successful treatment of pseudomyxoma peritonei (PMP) through cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (CRS-HIPEC): a case report and literature review[J]. Int J Surg Case Rep, 2024, 119: 109656. |
| [49] | PADMANABHAN N, ISHIBASHI H, NISHIHARA K, et al. Complete pathological response of high grade appendicular neoplasm induced pseudomyxoma peritonei (PMP) after neoadjuvant intra-peritoneal chemotherapy: a case report[J]. Int J Surg Case Rep, 2020, 72: 117-121. |
| [50] | FENG F L, GAO Q X, WU Y, et al. Cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy vs cytoreductive surgery alone for intrahepatic cholangiocarcinoma with peritoneal metastases: a retrospective cohort study[J]. Eur J Surg Oncol, 2021, 47(9): 2363-2368. |
| [51] | KRYH-JENSEN C G, FRISTRUP C W, AINSWORTH A P, et al. What is long-term survival in patients with peritoneal metastasis from gastric, pancreatic, or colorectal cancer? A study of patients treated with systemic chemotherapy and pressurized intraperitoneal aerosol chemotherapy (PIPAC)[J]. Pleura Peritoneum, 2023, 8(4): 147-155. |
| [52] | JI Z H, PENG K W, YU Y, et al. Current status and future prospects of clinical trials on CRS+HIPEC for gastric cancer peritoneal metastases[J]. Int J Hyperthermia, 2017, 33(5): 562-570. |
| [53] |
GLEHEN O, GILLY F N, ARVIEUX C, et al. Peritoneal carcinomatosis from gastric cancer: a multi-institutional study of 159 patients treated by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy[J]. Ann Surg Oncol, 2010, 17(9): 2370-2377.
doi: 10.1245/s10434-010-1039-7 pmid: 20336386 |
| [54] | LÓPEZ-BASAVE H N, QUIROZ-SANDOVAL O A, PADILLA-ROSCIANO A E, et al. Papel de la cirugía citorreductora y la quimioterapia intraperitoneal hipertérmica en el tratamiento del cáncer gástrico[J]. Cirugía Y Cirujanos, 2019, 86(3): 936. |
| [55] |
QUÉNET F, ELIAS D, ROCA L, et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy versus cytoreductive surgery alone for colorectal peritoneal metastases (PRODIGE 7): a multicentre, randomised, open-label, phase 3 trial[J]. Lancet Oncol, 2021, 22(2): 256-266.
doi: 10.1016/S1470-2045(20)30599-4 pmid: 33476595 |
| [56] | National Comprehensive Cancer Network. NCCN guideline in ovarian cancer/fallopian tube cancer/primary peritoneal cancer (version 1. 2022)[EB/OL]. (2022-03-02) [2024-07-31]. http://www,nccn,org/professionals/physician gls/pdf/ovarian.pdf . |
| [57] | VAN DRIEL W J, KOOLE S N, SIKORSKA K, et al. Hyperthermic intraperitoneal chemotherapy in ovarian cancer[J]. N Engl J Med, 2018, 378(3): 230-240. |
| [58] |
ARONSON S L, LOPEZ-YURDA M, KOOLE S N, et al. Cytoreductive surgery with or without hyperthermic intraperitoneal chemotherapy in patients with advanced ovarian cancer (OVHIPEC-1): final survival analysis of a randomised, controlled, phase 3 trial[J]. Lancet Oncol, 2023, 24(10): 1109-1118.
doi: 10.1016/S1470-2045(23)00396-0 pmid: 37708912 |
| [59] |
SUGARBAKER P H. New standard of care for appendiceal epithelial neoplasms and pseudomyxoma peritonei syndrome?[J]. Lancet Oncol, 2006, 7(1): 69-76.
doi: 10.1016/S1470-2045(05)70539-8 pmid: 16389186 |
| [60] |
CHUA T C, MORAN B J, SUGARBAKER P H, et al. Early- and long-term outcome data of patients with pseudomyxoma peritonei from appendiceal origin treated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy[J]. J Clin Oncol, 2012, 30(20): 2449-2456.
doi: 10.1200/JCO.2011.39.7166 pmid: 22614976 |
| [61] |
SUGARBAKER P H, VAN DER SPEETEN K. Surgical technology and pharmacology of hyperthermic perioperative chemotherapy[J]. J Gastrointest Oncol, 2016, 7(1): 29-44.
doi: 10.3978/j.issn.2078-6891.2015.105 pmid: 26941982 |
| [62] | BREUSA S, ZILIO S, CATANIA G, et al. Localized chemotherapy approaches and advanced drug delivery strategies: a step forward in the treatment of peritoneal carcinomatosis from ovarian cancer[J]. Front Oncol, 2023, 13: 1125868. |
| [63] |
LAGAST N, CARLIER C, CEELEN W P. Pharmacokinetics and tissue transport of intraperitoneal chemotherapy[J]. Surg Oncol Clin N Am, 2018, 27(3): 477-494.
doi: S1055-3207(18)30013-9 pmid: 29935684 |
| [64] |
MATSUMURA Y, MAEDA H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs[J]. Cancer Res, 1986, 46(12 Pt 1): 6387-6392.
pmid: 2946403 |
| [65] | SMET L D, CEELEN W, REMON J P, et al. Optimization of drug delivery systems for intraperitoneal therapy to extend the residence time of the chemotherapeutic agent[J]. Sci World J, 2013, 2013: 720858. |
| [66] | WANG X H, ZHANG H, CHEN X H, et al. Overcoming tumor microenvironment obstacles: current approaches for boosting nanodrug delivery[J]. Acta Biomater, 2023, 166: 42-68. |
| [67] | SUN T, ZHANG G P, NING T T, et al. A versatile theranostic platform for colorectal cancer peritoneal metastases: real-time tumor-tracking and photothermal-enhanced chemotherapy[J]. Adv Sci, 2021, 8(20): e2102256. |
| [68] | LIANG S, XIAO L Y, CHEN T, et al. Injectable nanocomposite hydrogels improve intraperitoneal co-delivery of chemotherapeutics and immune checkpoint inhibitors for enhanced peritoneal metastasis therapy[J]. ACS Nano, 2024, 18(29): 18963-18979. |
| [69] | MELAMED J R, YERNENI S S, ARRAL M L, et al. Ionizable lipid nanoparticles deliver mRNA to pancreatic β cells via macrophage-mediated gene transfer[J]. Sci Adv, 2023, 9(4): eade1444. |
| [70] | GU K, LIANG T, HU L, et al. Intraperitoneal programming of tailored CAR macrophages via mRNA-LNP to boost cancer immunotherapy[M/OL]. (2024-07-30) [2024-08-26]. http://biorxiv.org/lookup/doi/10.1101/2024.07.30.605730 . |
| [71] | CHEN Z M, TIAN Y, YANG J Y, et al. Modular design of biodegradable ionizable lipids for improved mRNA delivery and precise cancer metastasis delineation in vivo[J]. J Am Chem Soc, 2023, 145(44): 24302-24314. |
| [72] | HABER T, CORNEJO Y R, ARAMBURO S, et al. Specific targeting of ovarian tumor-associated macrophages by large, anionic nanoparticles[J]. Proc Natl Acad Sci U S A, 2020, 117(33): 19737-19745. |
| [73] | QU H J, YANG J F, LI S J, et al. Programmed-response cross-linked nanocarrier for multidrug-resistant ovarian cancer treatment[J]. J Control Release, 2023, 357: 274-286. |
| [74] | WANG K, SHEN R Y, MENG T T, et al. Nano-drug delivery systems based on different targeting mechanisms in the targeted therapy of colorectal cancer[J]. Molecules, 2022, 27(9): 2981. |
| [75] | HAN X J, GONG C N, YANG Q R, et al. Biomimetic nano-drug delivery system: an emerging platform for promoting tumor treatment[J]. Int J Nanomedicine, 2024, 19: 571-608. |
| [76] | ZAIKI Y, ISKANDAR A, WONG T W. Functionalized chitosan for cancer nano drug delivery[J]. Biotechnol Adv, 2023, 67: 108200. |
| [1] | CAO Xiaoshan, YANG Beibei, CONG Binbin, LIU Hong. The progress of treatment for brain metastases of triple-negative breast cancer [J]. China Oncology, 2024, 34(8): 777-784. |
| [2] | HUANG Sijie, KANG Xun, LI Wenbin. Clinical research progress of intrathecal therapy in the treatment of leptomeningeal metastasis [J]. China Oncology, 2024, 34(7): 695-701. |
| [3] | XU Yonghu, XU Dazhi. Progress and prospects of gastric cancer treatment in the 21st century [J]. China Oncology, 2024, 34(3): 239-249. |
| [4] | CHEN Yifan, LI Ting, WANG Biyun. Research progress of CCR8 in tumor immunotherapy [J]. China Oncology, 2024, 34(3): 299-305. |
| [5] | JIN Yizi, LIN Mingxi, ZENG Cheng, GUO Qing, ZHANG Jian. Research advances in estrogen receptor low positive early breast cancer [J]. China Oncology, 2024, 34(10): 972-978. |
| [6] | LIU Xuerou, YANG Yumei, ZHAO Qian, RONG Xiangyu, LIU Wei, ZHENG Ruijie, PANG Jinlong, LI Xian, LI Shanshan. Research progress on the role of glutamine metabolism-related proteins in tumor metastasis [J]. China Oncology, 2024, 34(1): 97-103. |
| [7] | KANG Yinnan, CHEN Shun, XIE Youcheng, ZHENG Ying, HE Yujing, LI Chuyi, YU Xiaohui. Application and research progress of antibody drug conjugates in HER2 positive advanced gastric cancer [J]. China Oncology, 2023, 33(8): 790-800. |
| [8] | WU Jing, ZHOU Juan, SU Chunxia. Advances in fatty acid metabolism reprogramming of lung cancer [J]. China Oncology, 2023, 33(5): 517-526. |
| [9] | JIANG Jinling, ZHOU Chenfei, WANG Chao, ZHAO Liqin, WU Junwei, ZHANG Jun. Advanced progress in research and diagnosis of gastric cancer in 2022 [J]. China Oncology, 2023, 33(4): 303-314. |
| [10] | TIAN Xi, XU Wenhao, ZHU Shuxuan, AIHETAIMUJIANG•Anwaier, SU Jiaqi, YE Shiqi, QU Yuanyuan, SHI Guohai, ZHANG Hailiang, YE Dingwei. Advances in the research, diagnosis and treatment of renal cell carcinoma in 2022 [J]. China Oncology, 2023, 33(3): 191-200. |
| [11] | SU Chunxia, ZHOU Caicun. Important clinical research progress in lung cancer in 2022 [J]. China Oncology, 2023, 33(3): 218-227. |
| [12] | CAO Xiaoshan, CONG Binbin. The research progress of endocrine therapy combined with targeted therapy for triple-positive breast cancer [J]. China Oncology, 2023, 33(3): 288-292. |
| [13] | SHAO Zhibo, YANG Benlong, WU Jiong. Progress of important clinical trials of breast cancer in China in 2022 [J]. China Oncology, 2023, 33(2): 103-109. |
| [14] | XIA Lingfang, ZHU Jun, WU Xiaohua. The latest progress and prospect of gynecological tumor treatment at 2023 ESMO [J]. China Oncology, 2023, 33(11): 969-980. |
| [15] | ZHAO Bolun, ZHU Guannan. Advances in the treatment of advanced melanoma with NRAS gene mutation [J]. China Oncology, 2023, 33(10): 936-944. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
沪ICP备12009617
Powered by Beijing Magtech Co. Ltd
