Welcome to China Oncology,
China Oncology ›› 2023, Vol. 33 ›› Issue (5): 460-468.doi: 10.19401/j.cnki.1007-3639.2023.05.006
• Article • Previous Articles Next Articles
ZUO Xueliang1,2( ), CHEN Zhiqiang3, DONG Runyu1, WANG Zhixiong1, CAI Juan2,4(
), CHEN Zhiqiang3, DONG Runyu1, WANG Zhixiong1, CAI Juan2,4( )
)
Received:2022-10-26
Revised:2023-03-02
Online:2023-05-30
Published:2023-06-16
Contact:
CAI Juan
Share article
CLC Number:
ZUO Xueliang, CHEN Zhiqiang, DONG Runyu, WANG Zhixiong, CAI Juan. The value of combined detection of LDHA and PD-L1 in predicting the efficacy and prognosis of advanced gastric cancer patients treated with PD-1 inhibitor[J]. China Oncology, 2023, 33(5): 460-468.
Tab. 1
Correlation between LDHA expression and clinicopathological features of gastric cancer patients"
| Characteristic | LDHA low expression | LDHA high expression | χ2 value | P value |
|---|---|---|---|---|
| Gender | 0.021 | 0.886 | ||
| Female | 6 | 4 | ||
| Male | 23 | 17 | ||
| Age/year | 0.618 | 0.432 | ||
| <60 | 8 | 8 | ||
| ≥60 | 21 | 13 | ||
| Tumor location | 0.019 | 0.890 | ||
| Down | 16 | 12 | ||
| Upper/middle | 13 | 9 | ||
| ECOG performance score | 0.069 | 0.793 | ||
| 0-1 | 19 | 13 | ||
| ≥2 | 10 | 8 | ||
| HER2 expression | 0.114 | 0.735 | ||
| Negative | 27 | 19 | ||
| Positive | 2 | 2 | ||
| Metastasis sites | 0.618 | 0.432 | ||
| ≤2 | 21 | 13 | ||
| >2 | 8 | 8 | ||
| PD-L1 CPS | 1.439 | 0.230 | ||
| ≥5 | 10 | 4 | ||
| <5 | 19 | 17 | ||
| PD-1 inhibitor response | ||||
| PD | 5 | 15 | 16.811 | <0.001 |
| SD | 7 | 4 | ||
| PR | 17 | 2 |
Tab. 2
Correlation between the efficacy of PD-1 inhibitor and clinicopathological features of gastric cancer patients"
| Characteristic | Responder | Non-responder | χ2 value | P value |
|---|---|---|---|---|
| Gender | 0.764 | 0.382 | ||
| Female | 5 | 5 | ||
| Male | 14 | 26 | ||
| Age/year | 3.701 | 0.054 | ||
| <60 | 3 | 13 | ||
| ≥60 | 16 | 18 | ||
| Tumor location | 2.401 | 0.121 | ||
| Down | 8 | 20 | ||
| Upper/middle | 11 | 11 | ||
| ECOG performance score | 0.496 | 0.481 | ||
| 0-1 | 11 | 21 | ||
| ≥2 | 8 | 10 | ||
| HER2 expression | 0.312 | 0.577 | ||
| Negative | 18 | 28 | ||
| Positive | 1 | 3 | ||
| Metastasis sites | 3.701 | 0.054 | ||
| ≤2 | 16 | 18 | ||
| >2 | 3 | 13 | ||
| Immunotherapy line | 7.371 | 0.007 | ||
| First-line | 7 | 2 | ||
| Second-line and above | 12 | 29 | ||
| PD-L1 CPS | 13.585 | <0.001 | ||
| ≥5 | 11 | 3 | ||
| <5 | 8 | 28 | ||
| LDHA expression | 12.462 | <0.001 | ||
| Low | 17 | 12 | ||
| High | 2 | 19 |
Tab. 3
Multivariable logistic regression analysis of the efficacy of PD-1 inhibitor in gastric cancer patients"
| Characteristic | OR | 95% CI | P value |
|---|---|---|---|
| Immunotherapy line (second and above) | 19.757 | 0.945-413.003 | 0.054 |
| PD-L1 CPS<5 | 24.180 | 2.049-285.278 | 0.011 |
| LDHA high expression | 44.987 | 2.916-693.960 | 0.006 |
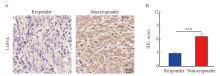
Fig. 1
LDHA expression is negatively correlated with the efficacy of PD-1 inhibitor in gastric cancer A: Representative IHC photographs of LDHA expression in tumor tissues from responders and non-responders. B: IHC scores of LDHA in tumor tissues from responders and non-responders. ***: P<0.001, compared with responder."
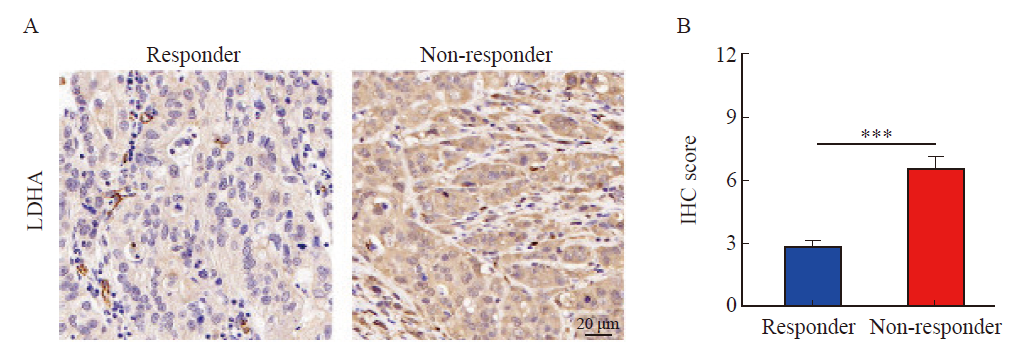

Fig. 2
ROC curve analyses of LDHA, PD-L1 and the combination of LDHA and PD-L1 in predicting the efficacy of PD-1 inhibitor in gastric cancer patients A: ROC curve of LDHA in predicting the efficacy of PD-1 inhibitor in gastric cancer patients; B: ROC curve of PD-L1 in predicting the efficacy of PD-1 inhibitor in gastric cancer patients; C: ROC curve of the combination of LDHA and PD-L1 in predicting the efficacy of PD-1 inhibitor in gastric cancer patients."
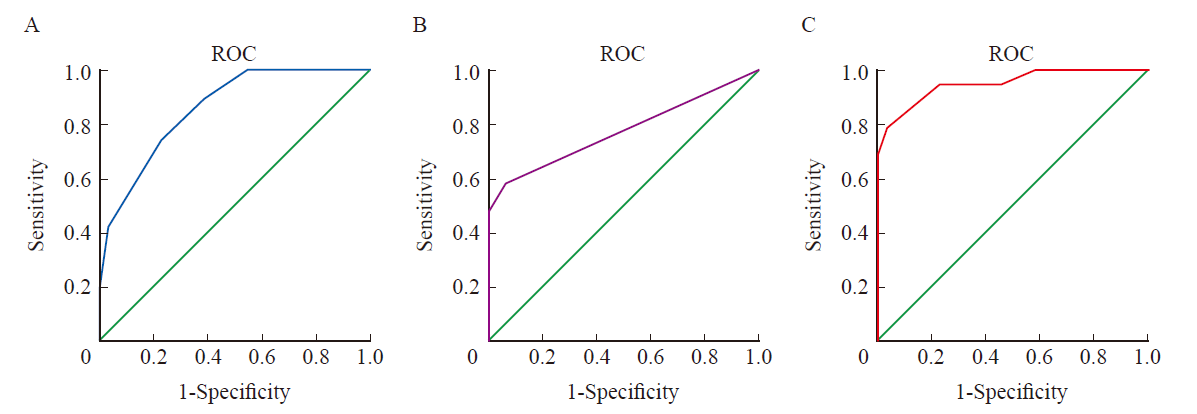

Fig. 3
Kaplan-Meier curve analyses of LDHA, PD-L1, the combination of LDHA and PD-L1 in predicting the OS of gastric cancer patients treated with PD-1 inhibitor A: Kaplan-Meier curve analysis of the correlation between LDHA expression and the OS of gastric cancer patients treated with PD-1 inhibitor; B: Kaplan-Meier curve analysis of the correlation between PD-L1 expression and the OS of gastric cancer patients treated with PD-1 inhibitor;C: Kaplan-Meier survival curves showing the effect of the combination of LDHA and PD-L1 on OS of gastric cancer patients treated with PD-1 inhibitor."
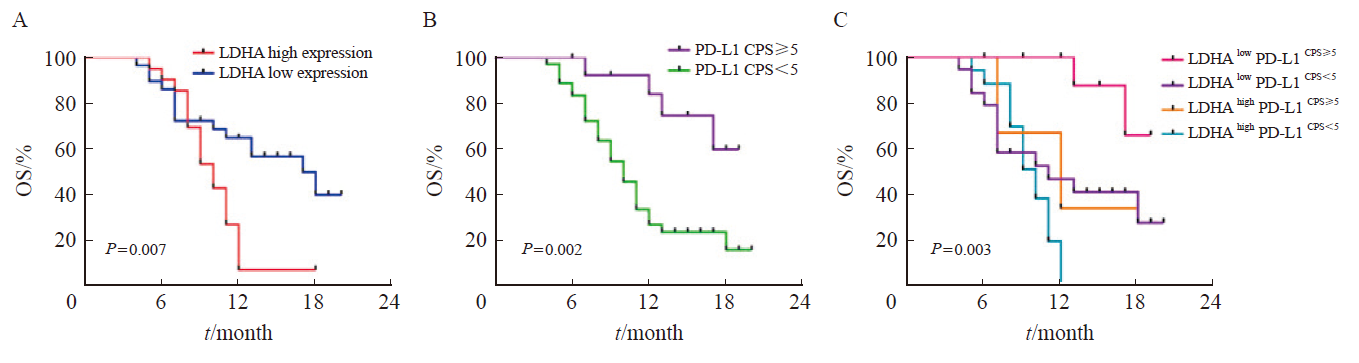

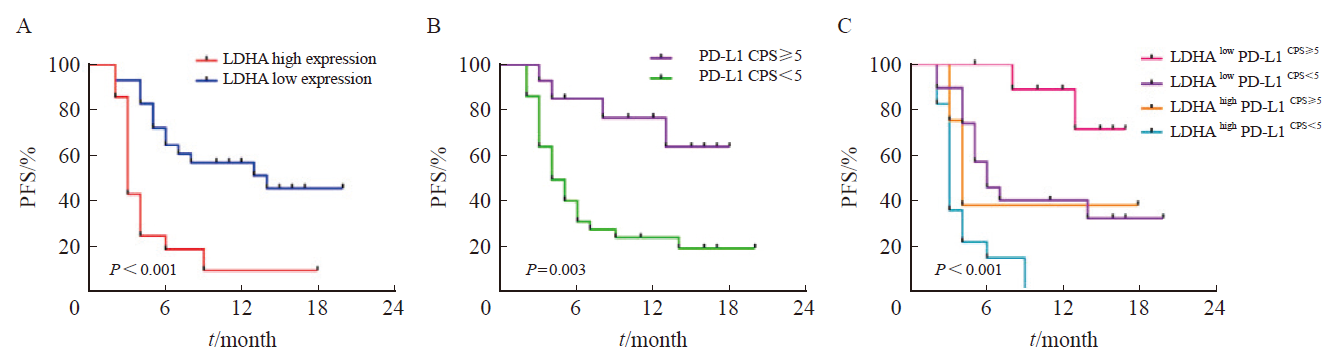
Fig. 4
Kaplan-Meier curve analyses of LDHA, PD-L1, the combination of LDHA and PD-L1 in predicting the PFS of gastric cancer patients treated with PD-1 inhibitor A: Kaplan-Meier curve analysis of the correlation between LDHA expression and the PFS of gastric cancer patients treated with PD-1 inhibitor; B: Kaplan-Meier curve analysis of the correlation between PD-L1 expression and the PFS of gastric cancer patients treated with PD-1 inhibitor; C: Kaplan-Meier survival curves showing the effect of the combination of LDHA and PD-L1 on PFS of gastric cancer patients treated with PD-1 inhibitor."
Tab. 4
Univariate analysis of OS and PFS of gastric cancer patients treated with PD-1 inhibitor"
| Characteristic | OS | PFS | ||
|---|---|---|---|---|
| Log-rank | P value | Log-rank | P value | |
| Gender (male vs female) | 0.085 | 0.770 | 0.060 | 0.806 |
| Age (≥60 years vs <60 years) | 1.914 | 0.167 | 3.063 | 0.080 |
| Tumor location (upper/middle vs down) | 1.034 | 0.309 | 0.847 | 0.357 |
| ECOG performance score (≥2 vs <2) | 1.302 | 0.254 | 0.894 | 0.344 |
| HER2 expression (positive vs negative) | 0.173 | 0.677 | 0.079 | 0.778 |
| Metastasis sites (>2 vs ≤2) | 5.195 | 0.023 | 5.744 | 0.017 |
| Immunotherapy line (first vs second and above) | 1.994 | 0.158 | 2.597 | 0.107 |
| PD-L1 CPS (<5 vs ≥5) | 9.334 | 0.002 | 9.004 | 0.003 |
| LDHA expression (high vs low) | 7.265 | 0.007 | 14.913 | <0.001 |
| [1] |
SUNG H, FERLAY J, SIEGEL R L, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2021, 71(3): 209-249.
doi: 10.3322/caac.v71.3 |
| [2] |
BAGCHI S, YUAN R, ENGLEMAN E G. Immune checkpoint inhibitors for the treatment of cancer: clinical impact and mechanisms of response and resistance[J]. Annu Rev Pathol, 2021, 16: 223-249.
doi: 10.1146/annurev-pathol-042020-042741 pmid: 33197221 |
| [3] |
JANJIGIAN Y Y, SHITARA K, MOEHLER M, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial[J]. Lancet, 2021, 398(10294): 27-40.
doi: 10.1016/S0140-6736(21)00797-2 pmid: 34102137 |
| [4] |
KANG Y K, CHEN L T, RYU M H, et al. Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (ATTRACTION-4): a randomised, multicentre, double-blind, placebo-controlled, phase 3 trial[J]. Lancet Oncol, 2022, 23(2): 234-247.
doi: 10.1016/S1470-2045(21)00692-6 |
| [5] |
SHITARA K, VAN CUTSEM E, BANG Y J, et al. Efficacy and safety of pembrolizumab or pembrolizumab plus chemotherapy vs chemotherapy alone for patients with first-line, advanced gastric cancer: the KEYNOTE-062 phase 3 randomized clinical trial[J]. JAMA Oncol, 2020, 6(10): 1571-1580.
doi: 10.1001/jamaoncol.2020.3370 |
| [6] |
SMYTH E C, NILSSON M, GRABSCH H I, et al. Gastric cancer[J]. Lancet, 2020, 396(10251): 635-648.
doi: S0140-6736(20)31288-5 pmid: 32861308 |
| [7] |
LIU Y, GUO J Z, LIU Y, et al. Nuclear lactate dehydrogenase A senses ROS to produce α-hydroxybutyrate for HPV-induced cervical tumor growth[J]. Nat Commun, 2018, 9(1): 4429.
doi: 10.1038/s41467-018-06841-7 pmid: 30356100 |
| [8] |
SUN X R, SUN Z, ZHU Z, et al. Clinicopathological significance and prognostic value of lactate dehydrogenase A expression in gastric cancer patients[J]. PLoS One, 2014, 9(3): e91068.
doi: 10.1371/journal.pone.0091068 |
| [9] |
GONG Y, JI P, YANG Y S, et al. Metabolic-pathway-based subtyping of triple-negative breast cancer reveals potential therapeutic targets[J]. Cell Metab, 2021, 33(1): 51-64.e9.
doi: 10.1016/j.cmet.2020.10.012 pmid: 33181091 |
| [10] | 中国临床肿瘤学会指南工作委员会. 中国临床肿瘤学会(CSCO)胃癌诊疗指南2022[M]. 北京: 人民卫生出版社, 2022. |
| Guidelines Working Committee of the Chinese Clinical Oncology Society. Chinese Society of Clinical Oncology (CSCO) guidelines for the diagnosis and treatment of gastric cancer 2022[M]. Beijing: People’s Health Publishing House, 2022. | |
| [11] |
DEY P, KIMMELMAN A C, DEPINHO R A. Metabolic codependencies in the tumor microenvironment[J]. Cancer Discov, 2021, 11(5): 1067-1081.
doi: 10.1158/2159-8290.CD-20-1211 pmid: 33504580 |
| [12] |
MARTÍNEZ-REYES I, CHANDEL N S. Cancer metabolism: looking forward[J]. Nat Rev Cancer, 2021, 21(10): 669-680.
doi: 10.1038/s41568-021-00378-6 |
| [13] |
LI X Y, WENES M, ROMERO P, et al. Navigating metabolic pathways to enhance antitumour immunity and immunotherapy[J]. Nat Rev Clin Oncol, 2019, 16(7): 425-441.
doi: 10.1038/s41571-019-0203-7 pmid: 30914826 |
| [14] |
VAUPEL P, SCHMIDBERGER H, MAYER A. The Warburg effect: essential part of metabolic reprogramming and central contributor to cancer progression[J]. Int J Radiat Biol, 2019, 95(7): 912-919.
doi: 10.1080/09553002.2019.1589653 pmid: 30822194 |
| [15] |
MA E H, VERWAY M J, JOHNSON R M, et al. Metabolic profiling using stable isotope tracing reveals distinct patterns of glucose utilization by physiologically activated CD8+ T cells[J]. Immunity, 2019, 51(5): 856-870.e5.
doi: 10.1016/j.immuni.2019.09.003 |
| [16] |
KAYMAK I, WILLIAMS K S, CANTOR J R, et al. Immunometabolic interplay in the tumor microenvironment[J]. Cancer Cell, 2021, 39(1): 28-37.
doi: 10.1016/j.ccell.2020.09.004 pmid: 33125860 |
| [17] |
JIANG W N, QIAO L, ZUO D, et al. Aberrant lactate dehydrogenase A signaling contributes metabolic signatures in pancreatic cancer[J]. Ann Transl Med, 2021, 9(4): 358.
doi: 10.21037/atm-21-295 pmid: 33708985 |
| [18] |
BRAND A, SINGER K, KOEHL G E, et al. LDHA-associated lactic acid production blunts tumor immunosurveillance by T and NK cells[J]. Cell Metab, 2016, 24(5): 657-671.
doi: S1550-4131(16)30427-2 pmid: 27641098 |
| [19] |
ZHANG A K, XU Y Z, XU H S, et al. Lactate-induced M2 polarization of tumor-associated macrophages promotes the invasion of pituitary adenoma by secreting CCL17[J]. Theranostics, 2021, 11(8): 3839-3852.
doi: 10.7150/thno.53749 pmid: 33664865 |
| [20] |
MIZRAHI J, PANT S. Immunotherapy in gastrointestinal malignancies[J]. Adv Exp Med Biol, 2020, 1244: 93-106.
doi: 10.1007/978-3-030-41008-7_5 pmid: 32301012 |
| [21] |
CHAO J, FUCHS C S, SHITARA K, et al. Assessment of pembrolizumab therapy for the treatment of microsatellite instability-high gastric or gastroesophageal junction cancer among patients in the KEYNOTE-059, KEYNOTE-061, and KEYNOTE-062 clinical trials[J]. JAMA Oncol, 2021, 7(6): 895-902.
doi: 10.1001/jamaoncol.2021.0275 pmid: 33792646 |
| [22] |
WAGNER N B, FORSCHNER A, LEITER U, et al. S100B and LDH as early prognostic markers for response and overall survival in melanoma patients treated with anti-PD-1 or combined anti-PD-1 plus anti-CTLA-4 antibodies[J]. Br J Cancer, 2018, 119(3): 339-346.
doi: 10.1038/s41416-018-0167-x |
| [23] |
DAHER S, LAWRENCE Y R, DUDNIK E, et al. Nivolumab in non-small cell lung cancer: real world long-term survival results and blood-based efficacy biomarkers[J]. Front Oncol, 2021, 11: 625668.
doi: 10.3389/fonc.2021.625668 |
| [24] |
QIAO T Y, XIONG Y L, FENG Y B, et al. Inhibition of LDH-a by oxamate enhances the efficacy of anti-PD-1 treatment in an NSCLC humanized mouse model[J]. Front Oncol, 2021, 11: 632364.
doi: 10.3389/fonc.2021.632364 |
| [25] |
ZHANG Y X, ZHAO Y Y, SHEN J Z, et al. Nanoenabled modulation of acidic tumor microenvironment reverses anergy of infiltrating T cells and potentiates anti-PD-1 therapy[J]. Nano Lett, 2019, 19(5): 2774-2783.
doi: 10.1021/acs.nanolett.8b04296 |
| [26] | FUCHS C S, DOI T, JANG R W, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial[J]. JAMA Oncol, 2018, 4(5): e180013. |
| [27] |
BANG Y J, KANG Y K, CATENACCI D V, et al. Pembrolizumab alone or in combination with chemotherapy as first-line therapy for patients with advanced gastric or gastroesophageal junction adenocarcinoma: results from the phase Ⅱ nonrandomized KEYNOTE-059 study[J]. Gastric Cancer, 2019, 22(4): 828-837.
doi: 10.1007/s10120-018-00909-5 |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
沪ICP备12009617
Powered by Beijing Magtech Co. Ltd
