Welcome to China Oncology,
China Oncology ›› 2024, Vol. 34 ›› Issue (4): 400-408.doi: 10.19401/j.cnki.1007-3639.2024.04.007
• Article • Previous Articles Next Articles
XU Yuchen1,2( ), ZHANG Jian1,2, WANG Yan3, LIN Jinyi1,2, ZHOU Yuhong3, CHENG Leilei2,4,5, GE Junbo1,2(
), ZHANG Jian1,2, WANG Yan3, LIN Jinyi1,2, ZHOU Yuhong3, CHENG Leilei2,4,5, GE Junbo1,2( )
)
Received:2024-01-18
Revised:2024-04-09
Online:2024-04-30
Published:2024-05-17
Contact:
GE Junbo
Share article
CLC Number:
XU Yuchen, ZHANG Jian, WANG Yan, LIN Jinyi, ZHOU Yuhong, CHENG Leilei, GE Junbo. Therapeutic effects of tofacitinib on steroid-resistant immune checkpoint inhibitor-associated myocarditis[J]. China Oncology, 2024, 34(4): 400-408.
Tab. 1
Baselinea characteristics and laboratory tests of comparator and tofacitinib group"
| Item | Comparator (n = 17) | Tofacitinib (n = 19) | P value | Item | Comparator (n = 17) | Tofacitinib (n = 19) | P value | |
|---|---|---|---|---|---|---|---|---|
| Patients’ characteristics | Cerebral vascular disease n(%) | 0.90 | ||||||
| Age at diagnosis/year | 64.2±9.7 | 64.1±9.7 | 0.93 | Yes | 3 (17.6) | 3 (15.8) | ||
| Gender n(%) | 0.87 | No | 14 (82.4) | 16 (84.2) | ||||
| Male | 13 (72.2) | 14 (73.7) | Autoimmune disease n(%) | - | ||||
| Female | 4 (27.8) | 5 (26.3) | Yes | 0 (0.0) | 1 (5.3) | |||
| Smoking n(%) | 0.66 | No | 17 (100.0) | 19 (94.7) | ||||
| Yes | 5 (29.4) | 7 (36.8) | Cancer type n(%) | |||||
| No | 12 (70.6) | 12 (63.2) | Gastroenteric cancer | 9 (52.9) | 7 (36.8) | 0.35 | ||
| Hypertension n(%) | 0.97 | Hepatoma | 5 (29.4) | 4 (21.1) | 0.58 | |||
| Yes | 7 (41.1) | 8 (42.1) | Genitourinary cancer | 0 (0.0) | 3 (15.8) | - | ||
| No | 10 (58.9) | 11 (57.9) | Skin | 1 (5.9) | 0 (0.0) | - | ||
| Diabetes mellitus n(%) | 0.90 | Other | 2 (17.6) | 5 (26.3) | 0.29 | |||
| Yes | 3 (17.6) | 3 (15.8) | Baseline laboratory tests | |||||
| No | 14 (82.4) | 16 (84.2) | Neutrophil absolute value (109/L) | 45.4±37.3 | 63.4±16.6 | 0.63 | ||
| Coronary artery disease n(%) | 0.75 | Lymphocyte absolute value (109/L) | 16.8±14.8 | 23.6±13.7 | 0.28 | |||
| Yes | 2 (11.8) | 3 (15.8) | Albumin/(g·L-1) | 40.7±7.5 | 40.0±5.4 | 1.00 | ||
| No | 15 (88.2) | 16 (84.2) | Platelet value (109/L) | 161.7±51.9 | 163.4±62.4 | 0.88 | ||
| Prior history of LV systolic dysfunction n(%) | - | CRP/(mg·L-1) | 9.0±5.8 | 7.7±4.1 | 0.79 | |||
| Yes | 0 (0.0) | 1 (5.3) | LDH/(U·L-1) | 137.3±29.7 | 276.0±108.8 | 0.057 | ||
| No | 17 (100.0) | 19 (94.7) | TNF-α/(nmol·L-1) | 2.12±0.1 | 1.43±0.2 | 0.095 |
Tab. 2
Clinical details and management of comparator and tofacitinib group"
| Item | Comparator (n=17) | Tofacitinib (n=19) | P value | Item | Comparator (n=17) | Tofacitinib (n=19) | P value | |
|---|---|---|---|---|---|---|---|---|
| ICI regimen n(%) | cTnT level, days following treatment | |||||||
| PD-1 inhibitors | cTnT of day 0 d/(ng·mL-1) | 0.55±0.44 | 0.30±0.26 | 0.043 | ||||
| Camrelizumab | 3 (18) | 6 (32) | 0.35 | cTnT of day 3/(ng·mL-1) | 0.50±0.51 | 0.29±0.33 | 0.16 | |
| Pembrolizumab | 2 (12) | 5 (43) | 0.29 | cTnT of day 7/(ng·mL-1) | 0.48±0.42 | 0.23±0.22 | 0.031 | |
| Toripalimab | 3 (18) | 2 (11) | 0.56 | cTnT of day 14/(ng·mL-1) | 0.42±0.36 | 0.16±0.17 | 0.008 | |
| Sintilimab | 3 (18) | 2 (11) | 0.56 | New ECG abnormality n(%) | ||||
| Tislelizumab | 3 (18) | 0 (0) | - | Atrial fibrillation | 1 (6) | 1 (5) | 0.97 | |
| Nivolumab | 0 (0) | 1 (5) | - | Supraventricular tachycardia | 0 (0) | 1 (5) | - | |
| PD-L1 inhibitors | Ventricular arrhythmia | 3 (18) | 2 (11) | 0.56 | ||||
| Durvalumab | 1 (6) | 0 (0) | - | High degree atrioventricular block | 3 (18) | 2 (11) | 0.56 | |
| ZKAB001 | 0 (0) | 1 (5) | - | New bundle branch block | 3 (18) | 3 (16) | 0.90 | |
| PD-1/CTLA-4 inhibitors | T wave or ST segment abnormality | 9 (53) | 7 (37) | 0.35 | ||||
| Candonilimab | 1 (6) | 2 (11) | 0.64 | Echocardiography | ||||
| Unknown a | 1 (6) | 0 (0) | - | LVEF (%) e | 60.6±9.4 | 61.5±8.1 | 0.79 | |
| Clinical presentation n(%) | New decline in LVEF n(%) | 4 (24) | 3 (16) | 0.58 | ||||
| Chest pain | 3 (18) | 2 (11) | 0.56 | Pericardial effusion n(%) | 1 (6) | 0 (0) | - | |
| Dyspnea | 8 (47) | 9 (47) | 1.00 | CMR n(%) | ||||
| Palpitations | 4 (24) | 3 (16) | 0.58 | CMR performed | 9 (53) | 12 (63) | 0.55 | |
| Myalgias | 6 (35) | 5 (26) | 0.58 | Myocarditis confirmed | 2 (12) | 2 (11) | 0.93 | |
| Weakness | 9 (53) | 10 (53) | 1.00 | Myocarditis suggestive | 7 (41) | 8 (42) | 0.97 | |
| Dizziness | 1 (6) | 3 (16) | 0.37 | Normal | 0 (0) | 2 (11) | - | |
| Diplopia and/or ptosis | 6 (35) | 5 (26) | 0.58 | Myocardial biopsy n(%) | 1 (6) | 2 (11) | 0.64 | |
| Concomitant irAE b n(%) | Pharmacological management n(%) | |||||||
| Myositis | 6 (35) | 5 (26) | 0.58 | Intravenous steroids | 17 (100) | 19 (100) | 1.00 | |
| Endocrine (thyroiditis or hypophysitis) | 3 (18) | 2 (11) | 0.56 | Additional immunosuppressive drugs | 9 (53) | 19 (100) | - | |
| Hepatitis | 4 (24) | 1 (5) | 0.12 | Intravenous immunoglobulin | 9 (53) | 0 (0) | - | |
| Pneumonitis | 2 (12) | 0 (0) | - | Infliximab | 2 (12) | 0 (0) | - | |
| Neuritis | 2 (12) | 1 (5) | 0.51 | Plasma exchange | 1 (6) | 0 (0) | - | |
| Grade of myocarditis c n(%) | Tofacitinib | 0 (0) | 19 (100) | - | ||||
| Grade 3 | 9 (53) | 9 (47) | 0.76 | |||||
| Grade 4 | 8 (47) | 10 (53) | 0.76 |
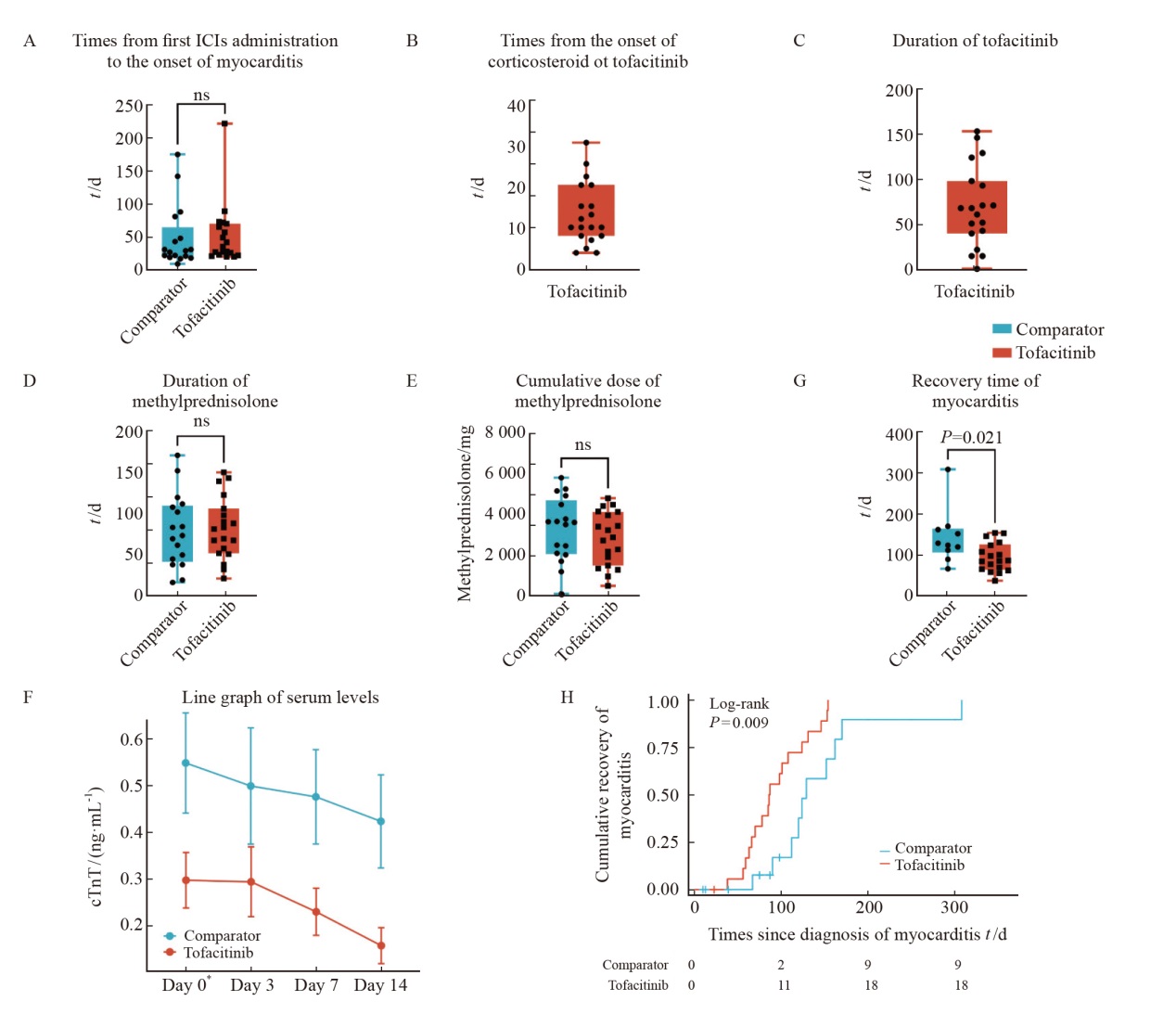
Fig. 2
Myocarditis presentation, clinical course and management A: Times from first ICIs administration to the onset of myocarditis; B: Times from the onset of corticosteroid to intensified immunosuppressive therapies with JAK inhibitors; C: Duration of tofacitinib; D-E: Duration and cumulative dose of methylprednisolone; F: The recovery time of patients in comparator group and tofacitinib group. G: Line graph of serum cTnT levels; H: Over time Kaplan-Meier curves of time to recovery of myocarditis comparator versus tofacitinib group; *: Day 0 means the day hormones are used. cTnT: Cardiac troponin T; ICI: Immune checkpoint inhibitor; ns: No significance."
| [1] | YANG K L, LI J R, ZHAO L, et al. Estimating the number of Chinese cancer patients eligible for and benefit from immune checkpoint inhibitors[J]. Front Med, 2022, 16(5): 773-783. |
| [2] | HASLAM A, PRASAD V. Estimation of the percentage of US patients with cancer who are eligible for and respond to checkpoint inhibitor immunotherapy drugs[J]. JAMA Netw Open, 2019, 2(5): e192535. |
| [3] |
CHHABRA N, KENNEDY J. A review of cancer immunotherapy toxicity: immune checkpoint inhibitors[J]. J Med Toxicol, 2021, 17(4): 411-424.
doi: 10.1007/s13181-021-00833-8 pmid: 33826117 |
| [4] |
WANG D Y, SALEM J E, COHEN J V, et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis[J]. JAMA Oncol, 2018, 4(12): 1721-1728.
doi: 10.1001/jamaoncol.2018.3923 pmid: 30242316 |
| [5] | HU J R, FLORIDO R, LIPSON E J, et al. Cardiovascular toxicities associated with immune checkpoint inhibitors[J]. Cardiovasc Res, 2019, 115(5): 854-868. |
| [6] | WANG C, LIN J Y, WANG Y, et al. Case series of steroid-resistant immune checkpoint inhibitor associated myocarditis: a comparative analysis of corticosteroid and tofacitinib treatment[J]. Front Pharmacol, 2021, 12: 770631. |
| [7] |
LYON A R, LÓPEZ-FERNÁNDEZ T, COUCH L S, et al. 2022 ESC guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS)[J]. Eur Heart J, 2022, 43(41): 4229-4361.
doi: 10.1093/eurheartj/ehac244 pmid: 36017568 |
| [8] | NGUYEN L S, BRETAGNE M, ARRONDEAU J, et al. Reversal of immune-checkpoint inhibitor fulminant myocarditis using personalized-dose-adjusted abatacept and ruxolitinib: proof of concept[J]. J Immunother Cancer, 2022, 10(4): e004699. |
| [9] |
KADOKAWA Y, TAKAGI M, YOSHIDA T, et al. Efficacy and safety of Infliximab for steroid-resistant immune-related adverse events: a retrospective study[J]. Mol Clin Oncol, 2021, 14(4): 65.
doi: 10.3892/mco.2021.2227 pmid: 33680456 |
| [10] |
ZHANG R S, PADEGIMAS A, MURPHY K M, et al. Treatment of corticosteroid refractory immune checkpoint inhibitor myocarditis with Infliximab: a case series[J]. Cardiooncology, 2021, 7(1): 13.
doi: 10.1186/s40959-021-00095-x pmid: 33785062 |
| [11] | XING Q, ZHANG Z W, ZHU B, et al. Case report: treatment for steroid-refractory immune-related myocarditis with tofacitinib[J]. Front Immunol, 2022, 13: 944013. |
| [12] |
YOGASUNDARAM H, ALHUMAID W, CHEN J W, et al. Plasma exchange for immune checkpoint inhibitor-induced myocarditis[J]. CJC Open, 2021, 3(3): 379-382.
doi: 10.1016/j.cjco.2020.11.004 pmid: 33778457 |
| [13] | IRVING P M, LEUNG Y, DUBINSKY M C. Review article: guide to tofacitinib dosing in patients with ulcerative colitis[J]. Aliment Pharmacol Ther, 2022, 56(7): 1131-1145. |
| [14] |
DHILLON S. Tofacitinib: a review in rheumatoid arthritis[J]. Drugs, 2017, 77(18): 1987-2001.
doi: 10.1007/s40265-017-0835-9 pmid: 29139090 |
| [15] |
PANÉS J, GISBERT J P. Efficacy of tofacitinib treatment in ulcerative colitis[J]. Gastroenterol Hepatol, 2019, 42(6): 403-412.
doi: S0210-5705(19)30094-9 pmid: 31101342 |
| [16] | OZDEDE A, YAZICI H. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis[J]. N Engl J Med, 2022, 386(18): 1766-1768. |
| [17] |
BONACA M P, OLENCHOCK B A, SALEM J E, et al. Myocarditis in the setting of cancer therapeutics: proposed case definitions for emerging clinical syndromes in cardio-oncology[J]. Circulation, 2019, 140(2): 80-91.
pmid: 31390169 |
| [18] |
EISENHAUER E A, THERASSE P, BOGAERTS J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1)[J]. Eur J Cancer, 2009, 45(2): 228-247.
doi: 10.1016/j.ejca.2008.10.026 pmid: 19097774 |
| [19] | SCHNEIDER B J, NAIDOO J, SANTOMASSO B D, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: ASCO guideline update[J]. J Clin Oncol, 2021, 39(36): 4073-4126. |
| [20] |
FRIEDRICH M G, SECHTEM U, SCHULZ-MENGER J, et al. Cardiovascular magnetic resonance in myocarditis: a JACC White Paper[J]. J Am Coll Cardiol, 2009, 53(17): 1475-1487.
doi: 10.1016/j.jacc.2009.02.007 pmid: 19389557 |
| [21] |
ARETZ H T. Myocarditis: the Dallas criteria[J]. Hum Pathol, 1987, 18(6): 619-624.
pmid: 3297992 |
| [22] |
MCDONAGH T A, METRA M, ADAMO M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC[J]. Eur J Heart Fail, 2022, 24(1): 4-131.
doi: 10.1002/ejhf.2333 pmid: 35083827 |
| [23] | SALEM J E, BRETAGNE M, ABBAR B, et al. Abatacept/ruxolitinib and screening for concomitant respiratory muscle failure to mitigate fatality of immune-checkpoint inhibitor myocarditis[J]. Cancer Discov, 2023, 13(5): 1100-1115. |
| [24] | FINKE D, HECKMANN M B, SALATZKI J, et al. Comparative transcriptomics of immune checkpoint inhibitor myocarditis identifies guanylate binding protein 5 and 6 dysregulation[J]. Cancers, 2021, 13(10): 2498. |
| [25] |
PHILIPS R L, WANG Y X, CHEON H, et al. The JAK-STAT pathway at 30: much learned, much more to do[J]. Cell, 2022, 185(21): 3857-3876.
doi: 10.1016/j.cell.2022.09.023 pmid: 36240739 |
| [26] |
TANAKA Y, LUO Y M, O’SHEA J J, et al. Janus kinase-targeting therapies in rheumatology: a mechanisms-based approach[J]. Nat Rev Rheumatol, 2022, 18(3): 133-145.
doi: 10.1038/s41584-021-00726-8 pmid: 34987201 |
| [27] | SANDBORN W J, SU C, SANDS B E, et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis[J]. N Engl J Med, 2017, 376(18): 1723-1736. |
| [28] | AXELROD M L, MEIJERS W C, SCREEVER E M, et al. T cells specific for α-myosin drive immunotherapy-related myocarditis[J]. Nature, 2022, 611(7937): 818-826. |
| [29] |
CHAPMAN N M, BOOTHBY M R, CHI H B. Metabolic coordination of T cell quiescence and activation[J]. Nat Rev Immunol, 2020, 20(1): 55-70.
doi: 10.1038/s41577-019-0203-y pmid: 31406325 |
| [30] |
DUSTIN M L. T-cell activation through immunological synapses and kinapses[J]. Immunol Rev, 2008, 221: 77-89.
doi: 10.1111/j.1600-065X.2008.00589.x pmid: 18275476 |
| [31] | HEINE A, HELD S A, DAECKE S N, et al. The JAK-inhibitor ruxolitinib impairs dendritic cell function in vitro and in vivo[J]. Blood, 2013, 122(7): 1192-1202. |
| [32] |
KUBO S, YAMAOKA K, KONDO M, et al. The JAK inhibitor, tofacitinib, reduces the T cell stimulatory capacity of human monocyte-derived dendritic cells[J]. Ann Rheum Dis, 2014, 73(12): 2192-2198.
doi: 10.1136/annrheumdis-2013-203756 pmid: 24013646 |
| [33] |
ZHANG H, LIN J Y, SHEN Y H, et al. Protective effect of crocin on immune checkpoint inhibitors-related myocarditis through inhibiting NLRP3 mediated pyroptosis in cardiomyocytes via NF-κB pathway[J]. J Inflamm Res, 2022, 15: 1653-1666.
doi: 10.2147/JIR.S348464 pmid: 35282269 |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
沪ICP备12009617
Powered by Beijing Magtech Co. Ltd
