Welcome to China Oncology,
China Oncology ›› 2022, Vol. 32 ›› Issue (10): 1007-1015.doi: 10.19401/j.cnki.1007-3639.2022.10.009
• Review • Previous Articles Next Articles
ZHANG Jiaxiang1( ), ZHOU Yongxue1(
), ZHOU Yongxue1( ), YAN Shuguang1, ZHAO Weihan2, DONG Fen3
), YAN Shuguang1, ZHAO Weihan2, DONG Fen3
Received:2022-01-26
Revised:2022-06-03
Online:2022-10-30
Published:2022-11-29
Share article
CLC Number:
ZHANG Jiaxiang, ZHOU Yongxue, YAN Shuguang, ZHAO Weihan, DONG Fen. Research progress of hypoxia-induced mitochondrial autophagy and glucose metabolism reprogramming in gastric precancerous lesions[J]. China Oncology, 2022, 32(10): 1007-1015.
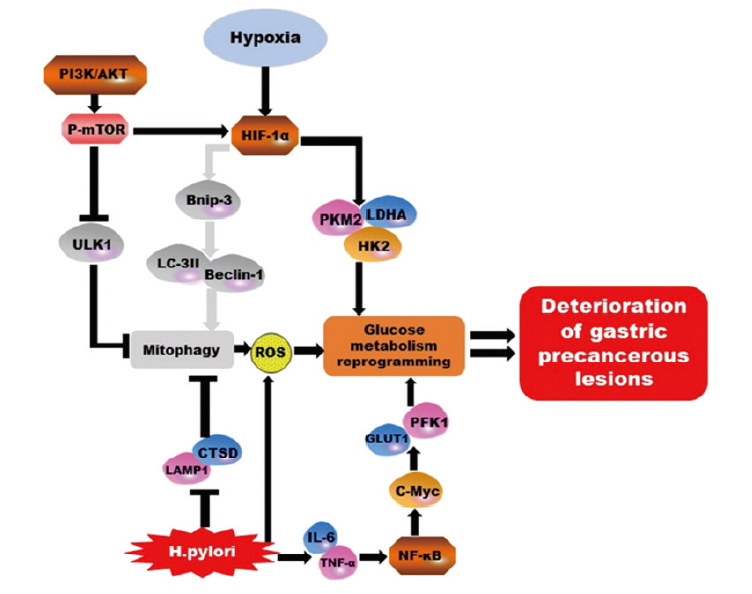
Fig. 1
A brief schematic diagram of the interaction principle between autophagy and glycolysis during GPL The PI3K/AKT/mTOR signaling pathway, H. pylori, ROS and hypoxia all inhibited autophagy or activated glycolysis through different mechanisms. The gray part in the figure showed the part that was inhibited or had no actual effect in the actual pathological process, and the black arrows indicated the actual changes."
| [1] | WANG S, KUANG J B, LI G F, et al. Gastric precancerous lesions present in ApcMin/+ mice[J]. Biomedecine Pharmacother, 2020, 121: 109534. |
| [2] |
GIATROMANOLAKI A, KOUKOURAKIS M I, KOUTSOPOULOS A V, et al. Autophagy and hypoxia in colonic adenomas related to aggressive features[J]. Colorectal Dis, 2013, 15(5): e223-e230.
doi: 10.1111/codi.12147 |
| [3] |
ZHANG C Z, CAI T T, ZENG X H, et al. Astragaloside Ⅳ reverses MNNG-induced precancerous lesions of gastric carcinoma in rats: regulation on glycolysis through miRNA-34a/LDHA pathway[J]. Phytother Res, 2018, 32(7): 1364-1372.
doi: 10.1002/ptr.6070 |
| [4] | 林翔英, 林翠丽, 田琳, 等. 脾胃湿热与胃癌前病变炎-癌转化机制的关系简析[J]. 中医杂志, 2021, 62(17): 1473-1477. |
| LIN X Y, LIN C L, TIAN L, et al. Relationship between spleen and stomach damp-heat and inflammation-cancer transformation mechanism of precancerous lesions of gastric cancer[J]. J Tradit Chin Med, 2021, 62(17): 1473-1477. | |
| [5] | 崔国良, 冯小可, 吴娟, 等. 化痰消瘀方治疗胃癌前病变的分子机制研究[J]. 中药药理与临床, 2021, 37(1): 172-179. |
| CUI G L, FENG X K, WU J, et al. Research on molecular mechanism of Huatan Xiaoyu Fang in the treatment of precancerous lesion of gastric cancer[J]. Pharmacol Clin Chin Mater Med, 2021, 37(1): 172-179. | |
| [6] | 唐翠娟, 荣震, 莫春梅, 等. 恶性肿瘤代谢特点及其与炎性介质的相关性研究进展[J]. 肿瘤防治研究, 2019, 46(5): 476-481. |
| TANG C J, RONG Z, MO C M, et al. Advances in metabolic characteristics of malignant tumors and their correlation with inflammatory mediators[J]. Cancer Res Prev Treat, 2019, 46(5): 476-481. | |
| [7] |
YANCOPOULOS G D, DAVIS S, GALE N W, et al. Vascular-specific growth factors and blood vessel formation[J]. Nature, 2000, 407(6801): 242-248.
doi: 10.1038/35025215 |
| [8] | 张成哲, 卓俊城, 蔡甜甜, 等. 胃炎1号方对胃癌前病变大鼠胃黏膜上皮细胞缺氧及缺氧耐受的影响[J]. 中成药, 2017, 39(5): 896-901. |
| ZHANG C Z, ZHUO J C, CAI T T, et al. Effects of No. 1 Weiyan Decoction on hypoxia and hypoxic tolerance in gastric mucosal epithelial cells in rats with gastric precancerous lesion[J]. Chin Tradit Pat Med, 2017, 39(5): 896-901. | |
| [9] | 刘莉菲, 仝晓阳, 郭健民, 等. HIF-1α在骨组织细胞代谢及骨疾病中的调控作用[J]. 中国细胞生物学学报, 2021, 43(2): 469-475. |
| LIU L F, TONG X Y, GUO J M, et al. Regulatory effects of HIF-1α in bone cell metabolism and bone diseases[J]. Chin J Cell Biol, 2021, 43(2): 469-475. | |
| [10] | 曾进浩, 潘华峰, 赵自明, 等. 健脾化瘀解毒复方治疗胃癌前病变的临床疗效及对HIF-1α、VEGF表达的影响[J]. 时珍国医国药, 2018, 29(7): 1544-1548. |
| ZENG J H, PAN H F, ZHAO Z M, et al. Clinical efficacy of Jianpi Huayu Jiedu compound in the treatment of precancerous lesions of gastric cancer and its effect on HIF-1 α, effect of VEGF expression[J]. Lishizhen Med Mater Med Res, 2018, 29(7): 1544-1548. | |
| [11] |
ESCOBAR K A, COLE N H, MERMIER C M, et al. Autophagy and aging: maintaining the proteome through exercise and caloric restriction[J]. Aging Cell, 2019, 18(1): e12876.
doi: 10.1111/acel.12876 |
| [12] | 田舍, 江建新, 喻超, 等. SIRT1通过调节FOXO1/RAB7信号通路促进低氧诱导的胰腺癌细胞自噬[J]. 中国病理生理杂志, 2019, 35(9): 1545-1550. |
| TIAN S, JIANG J X, YU C, et al. SIRT1 promotes autophagy of pancreatic cancer cells induced by hypoxia via regulating FOXO1/RAB7 signaling pathway[J]. Chin J Pathophysiol, 2019, 35(9): 1545-1550. | |
| [13] | 张刘杰, 李静, 吴博. HIF-1介导的自噬在心脏疾病中的研究进展[J]. 基础医学与临床, 2021, 41(8): 1186-1189. |
| ZHANG L J, LI J, WU B. Research progress of HIF-1 mediated autophagy in cardiac diseases[J]. Basic Clin Med, 2021, 41(8): 1186-1189. | |
| [14] |
VACEK J C, BEHERA J, GEORGE A K, et al. Tetrahydrocurcumin ameliorates homocysteine-mediated mitochondrial remodeling in brain endothelial cells[J]. J Cell Physiol, 2018, 233(4): 3080-3092.
doi: 10.1002/jcp.26145 pmid: 28833102 |
| [15] |
KRASSIKOVA L, ZHANG B X, NAGARAJAN D, et al. The deubiquitinase JOSD2 is a positive regulator of glucose metabolism[J]. Cell Death Differ, 2021, 28(3): 1091-1109.
doi: 10.1038/s41418-020-00639-1 pmid: 33082514 |
| [16] | 杨贺淳, 史道华. 调控肿瘤糖代谢重编程改善肿瘤耐药的小分子抑制剂研究进展[J]. 中国临床药理学与治疗学, 2021, 26(7): 836-840. |
| YANG H C, SHI D H. Research progress of small molecule inhibitors that reverse tumor drug resistance by regulating tumor glucose metabolism[J]. Chin J Clin Pharmacol Ther, 2021, 26(7): 836-840. | |
| [17] | 史新萌, 刘玉萍, 瞿鼎, 等. 抑制HIF-1α表达的中药抗肿瘤活性成分研究进展[J]. 药学学报, 2021, 56(10): 2689-2719. |
| SHI X M, LIU Y P, QU D, et al. Research progress of anti-tumor components of traditional Chinese medicine inhibiting the expression of HIF-1α[J]. Acta Pharm Sin, 2021, 56(10): 2689-2719. | |
| [18] |
LI H S, ZHOU Y N, LI L, et al. HIF-1α protects against oxidative stress by directly targeting mitochondria[J]. Redox Biol, 2019, 25: 101109.
doi: 10.1016/j.redox.2019.101109 |
| [19] | TANG W, LONG T T, LI F F, et al. HIF-1α may promote glycolysis in psoriasis vulgaris via upregulation of CD147 and GLUT1[J]. 中南大学学报(医学版), 2021, 46(4): 333-344. |
| TANG W, LONG T T, LI F F, et al. HIF-1α may promote glycolysis in psoriasis vulgaris via upregulation of CD147 and GLUT1[J]. J Central South Univ Med Sci, 2021, 46(4): 333-344. | |
| [20] |
KOZLOV A M, LONE A, BETTS D H, et al. Lactate preconditioning promotes a HIF-1α-mediated metabolic shift from OXPHOS to glycolysis in normal human diploid fibroblasts[J]. Sci Rep, 2020, 10(1): 8388.
doi: 10.1038/s41598-020-65193-9 pmid: 32433492 |
| [21] |
NASSOUR J, RADFORD R, CORREIA A, et al. Autophagic cell death restricts chromosomal instability during replicative crisis[J]. Nature, 2019, 565(7741): 659-663.
doi: 10.1038/s41586-019-0885-0 |
| [22] | 朱飞叶, 谢冠群, 徐珊. 乐胃饮对胃癌前病变大鼠模型自噬基因Beclin1的影响[J]. 中华中医药杂志, 2017, 32(1): 282-284. |
| ZHU F Y, XIE G Q, XU S. Effects of Lewei Drink on autophagy gene Beclin1 in rats with precancerous lesion of gastric cancer[J]. China J Tradit Chin Med Pharm, 2017, 32(1): 282-284. | |
| [23] | 胡平, 吴娟, 田薇, 等. 化痰消瘀方对大鼠胃癌前病变的疗效及对自噬相关基因的影响[J]. 中药材, 2019, 42(11): 2687-2691. |
| HU P, WU J, TIAN W, et al. Efficacy of Huatan Xiaoyu Recipe on gastric precancerous lesions in rats and its effect on autophagy-related genes[J]. J Chin Med Mater, 2019, 42(11): 2687-2691. | |
| [24] |
TSUGAWA H, MORI H, MATSUZAKI J, et al. CAPZA1 determines the risk of gastric carcinogenesis by inhibiting Helicobacter pylori CagA-degraded autophagy[J]. Autophagy, 2019, 15(2): 242-258.
doi: 10.1080/15548627.2018.1515530 |
| [25] |
ZAMPERONE A, COHEN D, STEIN M, et al. Inhibition of polarity-regulating kinase PAR1b contributes to helicobacter pylori inflicted DNA double strand breaks in gastric cells[J]. Cell Cycle, 2019, 18(3): 299-311.
doi: 10.1080/15384101.2018.1560121 pmid: 30580666 |
| [26] |
CUI Q B, WANG J Q, ASSARAF Y G, et al. Modulating ROS to overcome multidrug resistance in cancer[J]. Drug Resist Updat, 2018, 41: 1-25.
doi: 10.1016/j.drup.2018.11.001 |
| [27] |
MATHEW R, KARP C M, BEAUDOIN B, et al. Autophagy suppresses tumorigenesis through elimination of p62[J]. Cell, 2009, 137(6): 1062-1075.
doi: 10.1016/j.cell.2009.03.048 pmid: 19524509 |
| [28] | 鲍丽雅, 黄婷婷, 赵艳, 等. 幽门螺杆菌毒力蛋白CagA对胃癌细胞线粒体自噬相关蛋白表达的影响[J]. 中国病原生物学杂志, 2019, 14(12): 1394-1397. |
| BAO L Y, HUANG T T, ZHAO Y, et al. Effect of the Helicobacter pylori virulence protein CagA on the expression of mitochondrial autophagyassociated proteins in gastric cancer cells[J]. J Pathog Biol, 2019, 14(12): 1394-1397. | |
| [29] | 解曼, 齐兴四, 李晓宇, 等. 肝移植受者幽门螺杆菌感染诊治的临床实践[J]. 中华肝胆外科杂志, 2021, 27(5): 331-334. |
| XIE M, QI X S, LI X Y, et al. Helicobacter pylori infection in liver transplant recipients[J]. Chin J Hepatobiliary Surg, 2021, 27(5): 331-334. | |
| [30] |
RAJU D, HUSSEY S, ANG M, et al. Vacuolating cytotoxin and variants in Atg16L1 that disrupt autophagy promote Helicobacter pylori infection in humans[J]. Gastroenterology, 2012, 142(5): 1160-1171.
doi: 10.1053/j.gastro.2012.01.043 pmid: 22333951 |
| [31] | RONG L, LI Z D, LENG X, et al. Salidroside induces apoptosis and protective autophagy in human gastric cancer AGS cells through the PI3K/Akt/mTOR pathway[J]. Biomedecine Pharmacother, 2020, 122: 109726. |
| [32] | 潘华峰, 袁冬生, 刘伟, 等. 健脾化瘀解毒方抑制PI3K/AKT/HIF-1α通路阻断胃癌前病变恶性进展的机制[J]. 中华中医药杂志, 2020, 35(6): 2786-2790. |
| PAN H F, YUAN D S, LIU W, et al. Mechanism of Jianpi Huayu Jiedu Formula on inhibition PI3K/AKT/HIF-1α pathway and blocking malignant progression of gastric precancerous lesion[J]. China J Tradit Chin Med Pharm, 2020, 35(6): 2786-2790. | |
| [33] | 张少泉, 倪向荣, 李鑫辉. 知母皂苷元通过AMPK-mTOR-ULK1途径抑制肾小球系膜基质合成和激活自噬治疗糖尿病肾病的研究[J]. 现代中西医结合杂志, 2021, 30(11): 1180-1186. |
| ZHANG S Q, NI X R, LI X H. Sarsasapogenin inhibits glomerular mesangial matrix synthesis and activates autophagy to improve diabetic nephropathy through the AMPK-mTOR-ULK1 pathway[J]. Mod J Integr Tradit Chin West Med, 2021, 30(11): 1180-1186. | |
| [34] | 孙阳, 孙悦, 顾媛媛, 等. mTOR信号通路在细胞自噬和凋亡调节中的作用[J]. 中国医学装备, 2021, 18(1): 162-166. |
| SUN Y, SUN Y, GU Y Y, et al. Role of mTOR signaling pathway in the regulation of autophagy and apoptosis[J]. China Med Equip, 2021, 18(1): 162-166. | |
| [35] | 陈林波, 马凯丽, 陈佺, 等. 线粒体自噬的分子机制[J]. 中国科学: 生命科学, 2019, 49(9): 1045-1053. |
|
CHEN L B, MA K L, CHEN Q, et al. Mechanism of mitophagy in cell homeostasis[J]. Sci Sin Vitae, 2019, 49(9): 1045-1053.
doi: 10.1360/SSV-2019-0158 |
|
| [36] | 何嘉慧, 王熙才, 王佶, 等. 肿瘤微环境与乳腺癌细胞糖代谢重编程研究进展[J]. 肿瘤学杂志, 2021, 27(5): 359-363. |
| HE J H, WANG X C, WANG J, et al. Progress on metabolism reprogramming in tumor microenvironment of breast cancer[J]. J Chin Oncol, 2021, 27(5): 359-363. | |
| [37] |
PANIERI E, SANTORO M M. ROS homeostasis and metabolism: a dangerous liason in cancer cells[J]. Cell Death Dis, 2016, 7(6): e2253.
doi: 10.1038/cddis.2016.105 |
| [38] | SABBAH H N. Targeting the mitochondria in heart failure[J]. JACC Basic Transl Sci, 2020, 5(1): 88-106. |
| [39] |
LIU W, ZHAO Z M, LIU Y L, et al. Weipiling ameliorates gastric precancerous lesions in Atp4a-/- mice[J]. BMC Complement Altern Med, 2019, 19(1): 318.
doi: 10.1186/s12906-019-2718-y |
| [40] |
XIE Y, LIU L. Analysis of correlation between HP infection and activation of PI3K/Akt pathway in mucosal tissues of gastric cancer and precancerous lesions[J]. Oncol Lett, 2018, 16(5): 5615-5620.
doi: 10.3892/ol.2018.9329 pmid: 30344716 |
| [41] |
WOO Y M, SHIN Y, LEE E J, et al. Inhibition of aerobic glycolysis represses Akt/mTOR/HIF-1α axis and restores tamoxifen sensitivity in antiestrogen-resistant breast cancer cells[J]. PLoS One, 2015, 10(7): e0132285.
doi: 10.1371/journal.pone.0132285 |
| [42] |
LUKACOVA S, SØRENSEN B S, ALSNER J, et al. The impact of hypoxia on the activity of lactate dehydrogenase in two different pre-clinical tumour models[J]. Acta Oncol, 2008, 47(5): 941-947.
pmid: 17906983 |
| [43] |
LIN L, HUANG H, LIAO W, et al. MACC1 supports human gastric cancer growth under metabolic stress by enhancing the Warburg effect[J]. Oncogene, 2015, 34(21): 2700-2710.
doi: 10.1038/onc.2014.204 pmid: 25043301 |
| [44] | 喻俊榕, 郝彦伟, 程敬, 等. 基于MCT4/CD147探讨四君子汤加减改善酸性微环境逆转胃癌前病变的效应机制[J]. 中国实验方剂学杂志, 2021, 27(6): 30-36. |
| YU J R, HAO Y W, CHENG J, et al. Explore mechanism of modified Si junzitang in improving acidic microenvironment and reversing gastric precancerous lesions based on MCT4/CD147[J]. Chin J Exp Tradit Med Formulae, 2021, 27(6): 30-36. | |
| [45] |
JIAO L, ZHANG H L, LI D D, et al. Regulation of glycolytic metabolism by autophagy in liver cancer involves selective autophagic degradation of HK2 (hexokinase 2)[J]. Autophagy, 2018, 14(4): 671-684.
doi: 10.1080/15548627.2017.1381804 pmid: 28980855 |
| [46] | 郑惠之, 赵荣, 杨梅, 等. 龙胆泻肝汤联合三联疗法对Hp感染慢性胃炎患者血清PG及IL-8表达的影响[J]. 中国中西医结合消化杂志, 2022, 30(2): 81-84. |
| ZHENG H Z, ZHAO R, YANG M, et al. Effect of Longdan Xiegan Decoction combined with triple therapy on serum PG and IL-8 expression in patients with chronic gastritis infected by Hp[J]. Chin J Integr Tradit West Med Dig, 2022, 30(2): 81-84. | |
| [47] |
CAPURRO M I, GREENFIELD L K, PRASHAR A, et al. VacA generates a protective intracellular reservoir for Helicobacter pylori that is eliminated by activation of the lysosomal calcium channel TRPML1[J]. Nat Microbiol, 2019, 4(8): 1411-1423.
doi: 10.1038/s41564-019-0441-6 pmid: 31110360 |
| [48] | 程雪, 杜运秋, 张锐清, 等. HK2对幽门螺杆菌诱导胃上皮细胞GES-1自噬的影响[J]. 中国病原生物学杂志, 2020, 15(5): 522-526. |
| CHENG X, DU Y Q, ZHANG R Q, et al. The effect of HK2 on the autophagy of GES-1 gastric epithelial cells induced by Helicobacter pylori[J]. J Pathog Biol, 2020, 15(5): 522-526. | |
| [49] |
CHOURASIA A H, TRACY K, FRANKENBERGER C, et al. Mitophagy defects arising from BNip3 loss promote mammary tumor progression to metastasis[J]. EMBO Rep, 2015, 16(9): 1145-1163.
doi: 10.15252/embr.201540759 pmid: 26232272 |
| [50] |
JITSCHIN R, BÖTTCHER M, SAUL D, et al. Inflammation-induced glycolytic switch controls suppressivity of mesenchymal stem cells via STAT1 glycosylation[J]. Leukemia, 2019, 33(7): 1783-1796.
doi: 10.1038/s41375-018-0376-6 pmid: 30679801 |
| [51] |
SHIROKI T, YOKOYAMA M, TANUMA N, et al. Enhanced expression of the M2 isoform of pyruvate kinase is involved in gastric cancer development by regulating cancer-specific metabolism[J]. Cancer Sci, 2017, 108(5): 931-940.
doi: 10.1111/cas.13211 |
| [52] |
STRAUS D S. TNFα and IL-17 cooperatively stimulate glucose metabolism and growth factor production in human colorectal cancer cells[J]. Mol Cancer, 2013, 12(1): 78.
doi: 10.1186/1476-4598-12-78 |
| [53] | 嵇莹莹, 龚国清. PI3K/Akt/mTOR通路在炎症相关疾病中分子机制研究进展[J]. 药学研究, 2018, 37(4): 226-229. |
| JI Y Y, GONG G Q. Progress in molecular mechanism of PI3K/Akt/mTOR pathway in inflammation related diseases[J]. J Pharm Res, 2018, 37(4): 226-229. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
沪ICP备12009617
Powered by Beijing Magtech Co. Ltd
