Welcome to China Oncology,
China Oncology ›› 2023, Vol. 33 ›› Issue (6): 551-559.doi: 10.19401/j.cnki.1007-3639.2023.06.001
• Specialists' Commentary • Previous Articles Next Articles
WU Siyu( ), LI Junjie, SHAO Zhimin(
), LI Junjie, SHAO Zhimin( )
)
Received:2023-05-10
Revised:2023-06-01
Online:2023-06-30
Published:2023-07-26
Share article
CLC Number:
WU Siyu, LI Junjie, SHAO Zhimin. Development history and research progress of sentinel lymph node biopsy in breast cancer[J]. China Oncology, 2023, 33(6): 551-559.

Fig. 1
A timeline of the development history of SLNB in breast cancer SLNB: Sentinel lymph node biopsy; ALND: Axillary lymph node dissection; NAC: Neoadjuvant chemotherapy; MARI: Marking metastatic lymph node with radioactive iodine seeds; TAD: Targeted axillary dissection; RISAS: Radioactive iodine seed placement in the axilla with SLNB."
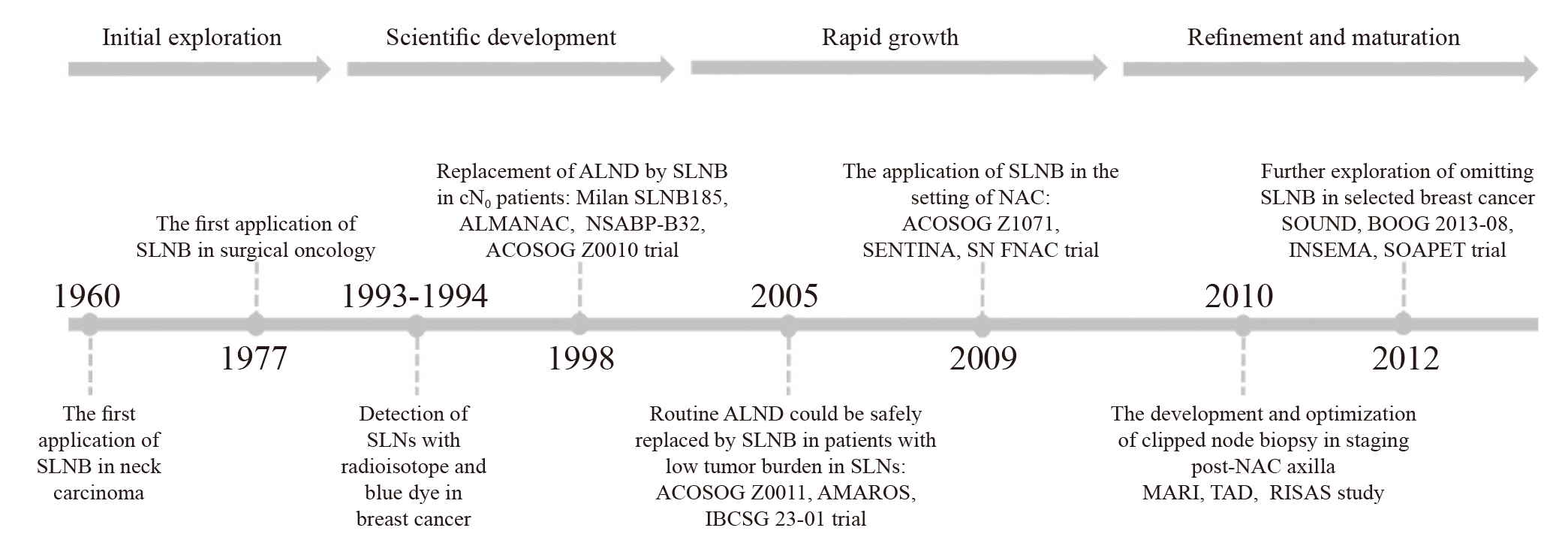
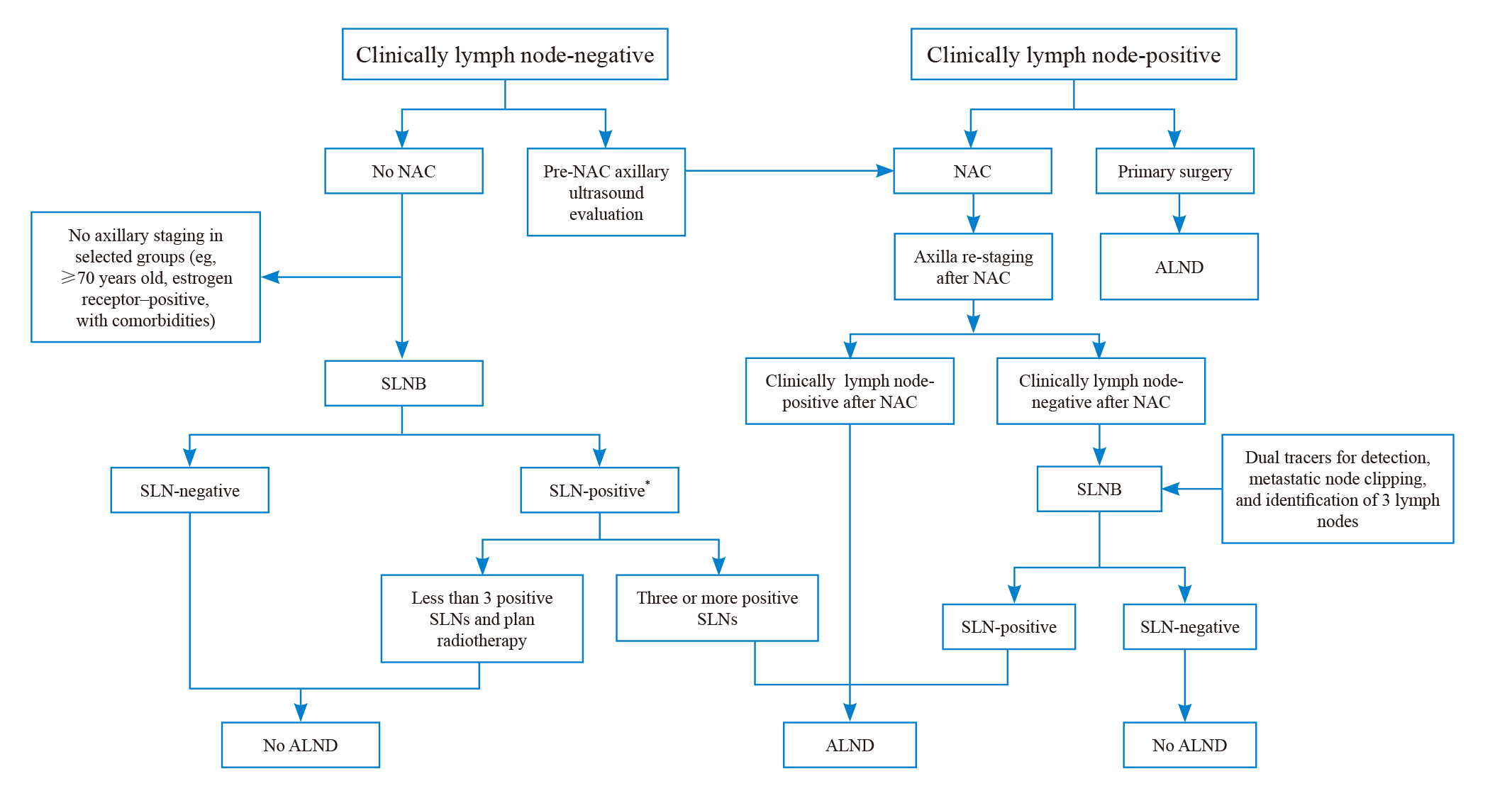
Fig. 2
Algorithm for the management of the axilla in patients with early-stage (stage Ⅰ to stage ⅡB) breast cancer *: In rare circumstances, such as T1aN1 stage, it is possible to avoid radiation. NAC: Neoadjuvant chemotherapy; SLNB: Sentinel lymph node biopsy; ALND: Axillary lymph node dissection; SLN: Sentinel lymph node."
| [1] | 马春雷, 斋登. 乳腺癌前哨淋巴结活检现状及研究进展[J]. 疾病监测与控制, 2017, 11(5): 380-382. |
| MA C L, ZHAI D. Current status and research progress of Sentinel lymph node biopsy in breast cancer[J]. Dis Monitoring Control, 2017, 11(5): 380-382. | |
| [2] | 周嵘, 曾繁余. 乳腺癌前哨淋巴结活检术的研究现状及展望[J]. 世界最新医学信息文摘, 2019, 19(43): 68-70. |
| ZHOU R, ZENG F Y. Research status and prospect of sentinel lymph node biopsy for breast cancer[J]. World Latest Med Inform Digest, 2019, 19(43): 68-70. | |
| [3] |
段学宁. 乳腺癌手术治疗百年历史回顾与启示[J]. 中国实用外科杂志, 2018, 38(11): 1227-1231.
doi: 10.19538/j.cjps.issn1005-2208.2018.11.03 |
| DUAN X N. Centennial review and enlightenment of breast cancer surgery[J]. China Ind Econ, 2018, 38(11): 1227-1231. | |
| [4] | 王金礼, 陈登峰. 乳腺癌前哨淋巴结活检术的研究进展[J]. 河南医学研究, 2017, 26(7): 1206-1208. |
| WANG J L. CHEN D F. Research progress of sentinel lymph node biopsy in breast cancer[J]. Henan Med Res, 2017, 26(7): 1206-1208. | |
| [5] |
KRAG D N, ANDERSON S J, JULIAN T B, et al. Technical outcomes of sentinel-lymph-node resection and conventional axillary-lymph-node dissection in patients with clinically node-negative breast cancer: results from the NSABP B-32 randomised phase Ⅲ trial[J]. Lancet Oncol, 2007, 8(10): 881-888.
doi: 10.1016/S1470-2045(07)70278-4 |
| [6] |
VERONESI U, PAGANELLI G, VIALE G, et al. A randomized comparison of sentinel-node biopsy with routine axillary dissection in breast cancer[J]. N Engl J Med, 2003, 349(6): 546-553.
doi: 10.1056/NEJMoa012782 |
| [7] |
MANSEL R E, FALLOWFIELD L, KISSIN M, et al. Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: the ALMANAC trial[J]. J Natl Cancer Inst, 2006, 98(9): 599-609.
doi: 10.1093/jnci/djj158 pmid: 16670385 |
| [8] |
PROF, DAVID N, KRAG, et al. Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: overall survival findings from the NSABP B-32 randomised phase 3 trial[J]. Lancet Oncol, 2010, 11(10): 927-933.
doi: 10.1016/S1470-2045(10)70207-2 pmid: 20863759 |
| [9] |
HUNT K K, BALLMAN K V, MCCALL L M, et al. Factors associated with local-regional recurrence after a negative sentinel node dissection: results of the ACOSOG Z0010 trial[J]. Ann Surg, 2012, 256(3): 428-436.
pmid: 22868365 |
| [10] |
LYMAN G H, GIULIANO A E, SOMERFIELD M R, et al. American Society of Clinical Oncology guideline recommendations for sentinel lymph node biopsy in early-stage breast cancer[J]. J Clin Oncol, 2005, 23(30): 7703-7720.
doi: 10.1200/JCO.2005.08.001 pmid: 16157938 |
| [11] |
GOLDHIRSCH A, WOOD W C, GELBER R D, et al. Progress and promise: highlights of the international expert consensus on the primary therapy of early breast cancer 2007[J]. Ann Oncol, 2007, 18(7): 1133-1144.
doi: 10.1093/annonc/mdm271 pmid: 17675394 |
| [12] | 王永胜, 欧阳涛, 王启堂, 等. 中国前哨淋巴结活检多中心协作研究CBCSG-001最新资料报告[J]. 中华乳腺病杂志(电子版), 2009, 3(3): 8-12. |
| WANG Y S, OUYANG T, WANG Q T, et al. Chinese joint multi-center study of sentinel lymph node biopsy (CBCSG-001): latest news[J]. Chin J Breast Dis Electron Version, 2009, 3(3): 8-12. | |
| [13] | 中国抗癌协会乳腺癌专业委员会. 中国抗癌协会乳腺癌诊治指南与规范(2021年版)[J]. 中国癌症杂志, 2021, 31(10): 954-1040. |
| The Society of Breast Cancer China Anti-Cancer Association. Guidelines for breast cancer diagnosis and treatment by China Anti-Cancer Association (2021 edition)[J]. China Oncol, 2021, 31(10): 954-1040. | |
| [14] |
VAN ZEE K J, MANASSEH D M E, BEVILACQUA J L B, et al. A nomogram for predicting the likelihood of additional nodal metastases in breast cancer patients with a positive sentinel node biopsy[J]. Ann Surg Oncol, 2003, 10(10): 1140-1151.
doi: 10.1245/aso.2003.03.015 pmid: 14654469 |
| [15] |
KOHRT H E, OLSHEN R A, BERMAS H R, et al. New models and online calculator for predicting non-sentinel lymph node status in sentinel lymph node positive breast cancer patients[J]. BMC Cancer, 2008, 8: 66.
doi: 10.1186/1471-2407-8-66 pmid: 18315887 |
| [16] |
GIULIANO A E, BALLMAN K V, MCCALL L, et al. Effect of axillary dissection vs no axillary dissection on 10-year overall survival among women with invasive breast cancer and sentinel node metastasis: the ACOSOG Z0011 (alliance) randomized clinical trial[J]. JAMA, 2017, 318(10): 918-926.
doi: 10.1001/jama.2017.11470 |
| [17] |
BARTELS S A L, DONKER M, PONCET C, et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer: 10-year results of the randomized controlled EORTC 10981-22023 AMAROS trial[J]. J Clin Oncol, 2023, 41(12): 2159-2165.
doi: 10.1200/JCO.22.01565 |
| [18] |
GALIMBERTI V, COLE B F, VIALE G, et al. Axillary dissection versus no axillary dissection in patients with breast cancer and sentinel-node micrometastases (IBCSG 23-01): 10-year follow-up of a randomised, controlled phase 3 trial[J]. Lancet Oncol, 2018, 19(10): 1385-1393.
doi: S1470-2045(18)30380-2 pmid: 30196031 |
| [19] |
PROF, THORSTEN, KUEHN, et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study[J]. Lancet Oncol, 2013, 14(7): 609-618.
doi: 10.1016/S1470-2045(13)70166-9 pmid: 23683750 |
| [20] |
BOUGHEY J C, SUMAN V J, MITTENDORF E A, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial[J]. JAMA, 2013, 310(14): 1455-1461.
doi: 10.1001/jama.2013.278932 pmid: 24101169 |
| [21] | BOILEAU J F, POIRIER B, BASIK M, et al. Sentinel node biopsy after neoadjuvant chemotherapy in biopsy-proven node-positive breast cancer: the SN FNAC study[J]. J Clin Oncol, 2015, 33(3): 258-264. |
| [22] |
BOUGHEY J C, SUMAN V J, MITTENDORF E A, et al. Factors affecting sentinel lymph node identification rate after neoadjuvant chemotherapy for breast cancer patients enrolled in ACOSOG Z1071 (Alliance)[J]. Ann Surg, 2015, 261(3): 547-552.
doi: 10.1097/SLA.0000000000000551 pmid: 25664534 |
| [23] | BOUGHEY J C, BALLMAN K V, LE-PETROSS H T, et al. Identification and resection of clipped node decreases the false-negative rate of sentinel lymph node surgery in patients presenting with node-positive breast cancer (T0-T4, N1-N2) who receive neoadjuvant chemotherapy: results from ACOSOG Z1071 (alliance)[J]. Ann Surg, 2016, 263(4): 802-807. |
| [24] |
DONKER M, STRAVER M E, WESSELING J, et al. Marking axillary lymph nodes with radioactive iodine seeds for axillary staging after neoadjuvant systemic treatment in breast cancer patients: the MARI procedure[J]. Ann Surg, 2015, 261(2): 378-382.
doi: 10.1097/SLA.0000000000000558 pmid: 24743607 |
| [25] |
CAUDLE A S, YANG W T, MITTENDORF E A, et al. Selective surgical localization of axillary lymph nodes containing metastases in patients with breast cancer: a prospective feasibility trial[J]. JAMA Surg, 2015, 150(2): 137-143.
doi: 10.1001/jamasurg.2014.1086 pmid: 25517573 |
| [26] |
CAUDLE A S, YANG W T, KRISHNAMURTHY S, et al. Improved axillary evaluation following neoadjuvant therapy for patients with node-positive breast cancer using selective evaluation of clipped nodes: implementation of targeted axillary dissection[J]. J Clin Oncol, 2016, 34(10): 1072-1078.
doi: 10.1200/JCO.2015.64.0094 pmid: 26811528 |
| [27] |
SIMONS J M, VAN NIJNATTEN T J A, VAN DER POL C C, et al. Diagnostic accuracy of radioactive iodine seed placement in the axilla with sentinel lymph node biopsy after neoadjuvant chemotherapy in node-positive breast cancer[J]. JAMA Surg, 2022, 157(11): 991-999.
doi: 10.1001/jamasurg.2022.3907 |
| [28] |
BRACKSTONE M, BALDASSARRE F G, PERERA F E, et al. Management of the axilla in early-stage breast cancer: Ontario health (cancer care Ontario) and ASCO guideline[J]. J Clin Oncol, 2021, 39(27): 3056-3082.
doi: 10.1200/JCO.21.00934 |
| [29] |
VUGTS G, MAASKANT-BRAAT A J, DE ROOS W K, et al. Management of the axilla after neoadjuvant chemotherapy for clinically node positive breast cancer: a nationwide survey study in the Netherlands[J]. Eur J Surg Oncol, 2016, 42(7): 956-964.
doi: 10.1016/j.ejso.2016.03.023 pmid: 27107791 |
| [30] |
CAUDLE A S, BEDROSIAN I, MILTON D R, et al. Use of sentinel lymph node dissection after neoadjuvant chemotherapy in patients with node-positive breast cancer at diagnosis: practice patterns of American Society of Breast Surgeons Members[J]. Ann Surg Oncol, 2017, 24(10): 2925-2934.
doi: 10.1245/s10434-017-5958-4 pmid: 28766207 |
| [31] |
WU S Y, WANG Y J, ZHANG N, et al. Intraoperative touch imprint cytology in targeted axillary dissection after neoadjuvant chemotherapy for breast cancer patients with initial axillary metastasis[J]. Ann Surg Oncol, 2018, 25(11): 3150-3157.
doi: 10.1245/s10434-018-6548-9 pmid: 30083833 |
| [32] |
WU S, WANG Y, LI J, et al. Subtype-guided 18F-FDG PET/CT in tailoring axillary surgery among patients with node-positive breast cancer treated with neoadjuvant chemotherapy: a feasibility study[J]. Oncologist, 2020, 25(4): e626-e633.
doi: 10.1634/theoncologist.2019-0583 |
| [33] |
WU S Y, LI J W, WU H L, et al. Accuracy of ultrasound-guided targeted fine-needle aspiration in assessing nodal response in node-positive breast cancer after neoadjuvant chemotherapy: prospective feasibility study[J]. Br J Surg, 2022, 109(12): 1194-1197.
doi: 10.1093/bjs/znac277 |
| [34] | WU S Y, LI J W, ZHANG Y, et al. Repeated core needle biopsy and targeted fine-needle aspiration to optimize axillary surgery after neoadjuvant chemotherapy in node-positive breast cancer: a prospective clinical study[J]. Br J Surg, 2023: znad106. |
| [35] |
PILTIN M A, HOSKIN T L, DAY C N, et al. Oncologic outcomes of sentinel lymph node surgery after neoadjuvant chemotherapy for node-positive breast cancer[J]. Ann Surg Oncol, 2020, 27(12): 4795-4801.
doi: 10.1245/s10434-020-08900-0 |
| [36] |
BARRIO A V, MONTAGNA G, MAMTANI A, et al. Nodal recurrence in patients with node-positive breast cancer treated with sentinel node biopsy alone after neoadjuvant chemotherapy-a rare event[J]. JAMA Oncol, 2021, 7(12): 1851-1855.
doi: 10.1001/jamaoncol.2021.4394 pmid: 34617979 |
| [37] |
MARTELLI G, BARRETTA F, MICELI R, et al. Sentinel node biopsy alone or with axillary dissection in breast cancer patients after primary chemotherapy: long-term results of a prospective interventional study[J]. Ann Surg, 2022, 276(5): e544-e552.
doi: 10.1097/SLA.0000000000004562 |
| [38] | WU S Y, LI J W, WANG Y J, et al. Clinical feasibility and oncological safety of non-radioactive targeted axillary dissection after neoadjuvant chemotherapy in biopsy-proven node-positive breast cancer: a prospective diagnostic and prognostic study[J]. Int J Surg, 2023. Online ahead of print. |
| [39] |
BURSTEIN H J, CURIGLIANO G, LOIBL S, et al. Estimating the benefits of therapy for early-stage breast cancer: the St. Gallen International Consensus Guidelines for the primary therapy of early breast cancer 2019[J]. Ann Oncol, 2019, 30(10): 1541-1557.
doi: S0923-7534(19)60978-6 pmid: 31987446 |
| [40] |
JATOI I, KUNKLER I H. Omission of sentinel node biopsy for breast cancer: historical context and future perspectives on a modern controversy[J]. Cancer, 2021, 127(23): 4376-4383.
doi: 10.1002/cncr.33960 pmid: 34614216 |
| [41] |
TADROS A B, YANG W T, KRISHNAMURTHY S, et al. Identification of patients with documented pathologic complete response in the breast after neoadjuvant chemotherapy for omission of axillary surgery[J]. JAMA Surg, 2017, 152(7): 665-670.
doi: 10.1001/jamasurg.2017.0562 pmid: 28423171 |
| [42] |
BARRON A U, HOSKIN T L, DAY C N, et al. Association of low nodal positivity rate among patients with ERBB2-positive or triple-negative breast cancer and breast pathologic complete response to neoadjuvant chemotherapy[J]. JAMA Surg, 2018, 153(12): 1120.
doi: 10.1001/jamasurg.2018.2696 pmid: 30193375 |
| [43] |
FAYANJU O M, REN Y, THOMAS S M, et al. The clinical significance of breast-only and node-only pathologic complete response (pCR) after neoadjuvant chemotherapy (NACT): a review of 20 000 breast cancer patients in the national cancer data base (NCDB)[J]. Ann Surg, 2018, 268(4): 591-601.
doi: 10.1097/SLA.0000000000002953 |
| [44] |
PFOB A, SIDEY-GIBBONS C, RAUCH G, et al. Intelligent vacuum-assisted biopsy to identify breast cancer patients with pathologic complete response (ypT0 and ypN0) after neoadjuvant systemic treatment for omission of breast and axillary surgery[J]. J Clin Oncol, 2022, 40(17): 1903-1915.
doi: 10.1200/JCO.21.02439 |
| [1] | XU Rui, WANG Zehao, WU Jiong. Advances in the role of tumor-associated neutrophils in the development of breast cancer [J]. China Oncology, 2024, 34(9): 881-889. |
| [2] | CAO Xiaoshan, YANG Beibei, CONG Binbin, LIU Hong. The progress of treatment for brain metastases of triple-negative breast cancer [J]. China Oncology, 2024, 34(8): 777-784. |
| [3] | ZHANG Jian. Clinical consideration of two key questions in assessing menopausal status of female breast cancer patients [J]. China Oncology, 2024, 34(7): 619-627. |
| [4] | JIANG Dan, SONG Guoqing, WANG Xiaodan. Study on the mechanism of mitochondrial dysfunction and CPT1A/ERK signal transduction pathway regulating malignant behavior in breast cancer [J]. China Oncology, 2024, 34(7): 650-658. |
| [5] | DONG Jianqiao, LI Kunyan, LI Jing, WANG Bin, WANG Yanhong, JIA Hongyan. A study on mechanism of SIRT3 inducing endocrine drug resistance in breast cancer via deacetylating YME1L1 [J]. China Oncology, 2024, 34(6): 537-547. |
| [6] | HAO Xian, HUANG Jianjun, YANG Wenxiu, LIU Jinting, ZHANG Junhong, LUO Yubei, LI Qing, WANG Dahong, GAO Yuwei, TAN Fuyun, BO Li, ZHENG Yu, WANG Rong, FENG Jianglong, LI Jing, ZHAO Chunhua, DOU Xiaowei. Establishment of primary breast cancer cell line as new model for drug screening and basic research [J]. China Oncology, 2024, 34(6): 561-570. |
| [7] | Committee of Breast Cancer Society, China Anti-Cancer Association. Expert consensus on clinical applications of ovarian function suppression for Chinese women with early breast cancer (2024 edition) [J]. China Oncology, 2024, 34(3): 316-333. |
| [8] | ZHANG Qi, XIU Bingqiu, WU Jiong. Progress of important clinical research of breast cancer in China in 2023 [J]. China Oncology, 2024, 34(2): 135-142. |
| [9] | ZHANG Siyuan, JIANG Zefei. Important research progress in clinical practice for advanced breast cancer in 2023 [J]. China Oncology, 2024, 34(2): 143-150. |
| [10] | WANG Zhaobu, LI Xing, YU Xinmiao, JIN Feng. Important research progress in clinical practice for early breast cancer in 2023 [J]. China Oncology, 2024, 34(2): 151-160. |
| [11] | LUO Yang, SUN Tao, SHAO Zhimin, CUI Jiuwei, PAN Yueyin, ZHANG Qingyuan, CHENG Ying, LI huiping, YANG Yan, YE Changsheng, YU Guohua, WANG Jingfen, LIU Yunjiang, LIU Xinlan, ZHOU Yuhong, BAI Yuju, GU Yuanting, WANG Xiaojia, XU Binghe, SONG Lihua. Efficacy, metabolic characteristics, safety and immunogenicity of AK-HER2 compared with reference trastuzumab in patients with metastatic HER2-positive breast cancer: a multicenter, randomized, double-blind phase Ⅲ equivalence trial [J]. China Oncology, 2024, 34(2): 161-175. |
| [12] | CHEN Yuanxiang, YU Tao, YANG Shiyu, ZENG Tao, WEI Lan, ZHANG Yan. KDM4A promotes the migration and invasion of breast cancer cell line MDA-MB-231 by downregulating BMP9 [J]. China Oncology, 2024, 34(2): 176-184. |
| [13] | HU Xiaoyu, CAI Yuwen, YE Fugui, SHAO Zhimin, HU Weigang, YU Keda. Impact of BRCA1/2 germline mutation on the incidence of second primary cancer following postoperative radiotherapy in patients with triple-negative breast cancer [J]. China Oncology, 2024, 34(2): 185-190. |
| [14] | ZHANG Siwei, MA Ding, JIANG Yizhou, SHAO Zhimin. “Subtype-precise” therapy leads diagnostic and therapeutic innovations: a new pattern for precision treatment of breast cancer [J]. China Oncology, 2024, 34(11): 1045-1052. |
| [15] | OUYANG Fei, WANG Yang, CHEN Yu, PEI Guoqing, WANG Ling, ZHANG Yang, SHI Lei. Construction of the prediction model of breast cancer bone metastasis based on machine learning [J]. China Oncology, 2024, 34(10): 903-914. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
沪ICP备12009617
Powered by Beijing Magtech Co. Ltd
