Welcome to China Oncology,
China Oncology ›› 2023, Vol. 33 ›› Issue (4): 377-387.doi: 10.19401/j.cnki.1007-3639.2023.04.008
• Article • Previous Articles Next Articles
LIU Yang1( ), HU Yiyang1, LIU Yueping2, NIU Shuyao2, DING Pingan1, TIAN Yuan1, GUO Honghai1, YANG Peigang1, ZHANG Ze1, ZHENG Tao1, TAN Bibo1, FAN Liqiao1, LI Yong1, ZHAO Qun1(
), HU Yiyang1, LIU Yueping2, NIU Shuyao2, DING Pingan1, TIAN Yuan1, GUO Honghai1, YANG Peigang1, ZHANG Ze1, ZHENG Tao1, TAN Bibo1, FAN Liqiao1, LI Yong1, ZHAO Qun1( )
)
Received:2022-05-27
Revised:2023-03-13
Online:2023-04-30
Published:2023-05-15
Contact:
ZHAO Qun
Share article
CLC Number:
LIU Yang, HU Yiyang, LIU Yueping, NIU Shuyao, DING Pingan, TIAN Yuan, GUO Honghai, YANG Peigang, ZHANG Ze, ZHENG Tao, TAN Bibo, FAN Liqiao, LI Yong, ZHAO Qun. The value of artificial intelligence-assisted technology in HER2 assessmentof gastric cancer patients receiving neoadjuvant chemotherapy[J]. China Oncology, 2023, 33(4): 377-387.
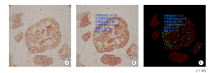
Fig. 1
AI-assisted microscope assessment A: Selected the area; B: Completed convolution network located tumor cells, delineated the stained membranes in different color; C: Shielded the signal of non-tumor cells and highlighted the stained cell membrane. The results showed that the complete and incomplete strong staining rates were 89.3% and 91.7%, the complete and incomplete weak staining rates were 0.0% and 0.0%, the no staining rate was 0.0%, suggested rating +++."
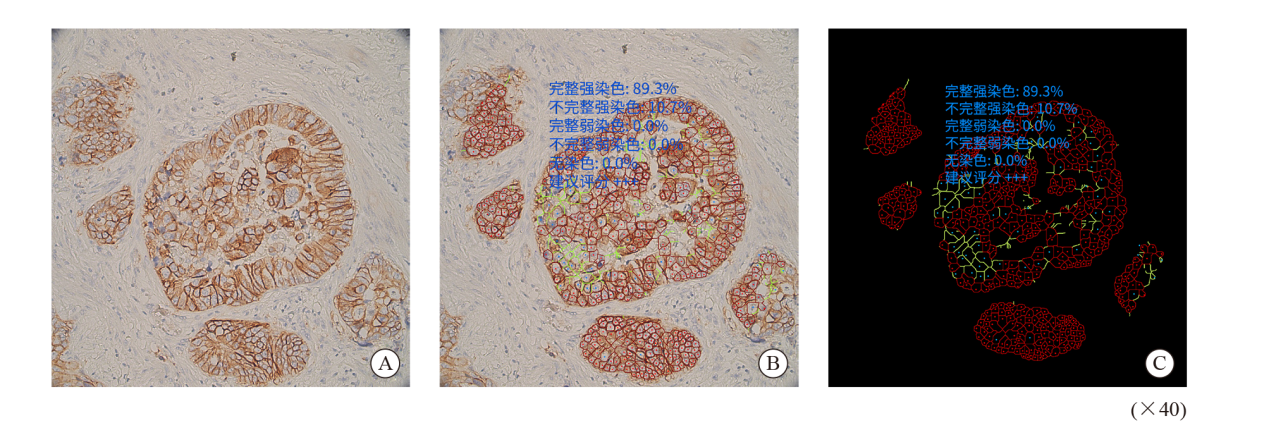
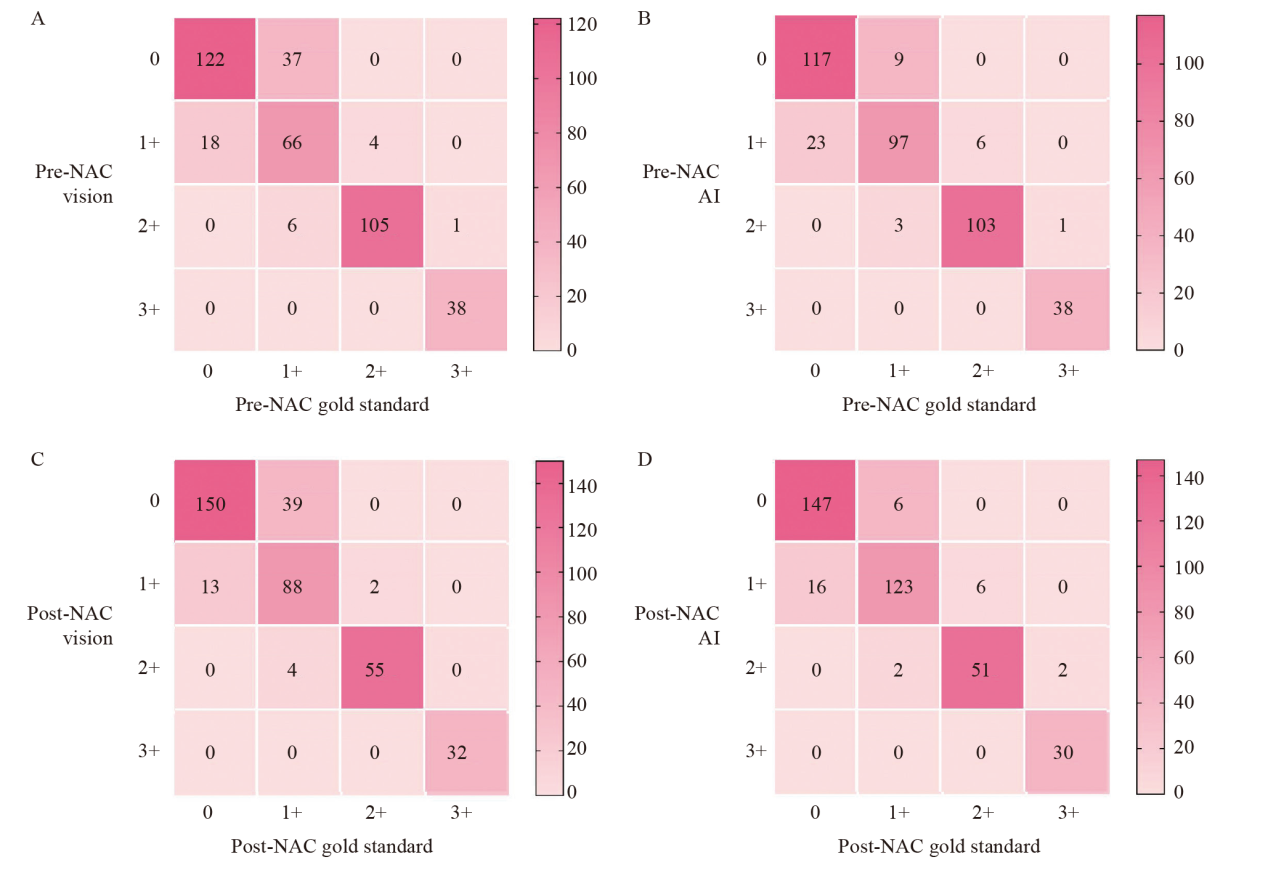
Fig. 4
Comparison of consistency between visual assessment, AI-assisted assessment and gold standard assessment A: Consistency of pre-NAC HER2 between visual assessment with gold standard (κ = 0.766); B: Consistency of pre-NAC HER2 between AI assessment with gold standard (κ = 0.853); C: Consistency of post-NAC HER2 between visual assessment with gold standard (κ = 0.773); D: Consistency of post-NAC HER2 between AI assessment with gold standard (κ = 0.876)."
Tab. 1
Changes in HER2 status after NAC assessed by AI and clinicopathologic factors"
| Clinicopathological parameter | HER2 expression changes/% | χ2 | P value | |
|---|---|---|---|---|
| Down-regulated expression (n=97) | Up-regulated expression (n=27) | |||
| Gender | 0.033 | 0.856 | ||
| Male | 77 (79.4) | 21 (77.8) | ||
| Female | 20 (20.6) | 6 (22.2) | ||
| Age/year | 0.808 | 0.369 | ||
| <60 | 48 (49.5) | 16 (59.3) | ||
| ≥60 | 49 (50.5) | 11 (40.7) | ||
| NEC regimen | 0.201 | 0.654 | ||
| XELOX | 55 (56.7) | 14 (51.9) | ||
| SOX | 42 (43.3) | 13 (48.1) | ||
| Tumor location | 1.050 | 0.592 | ||
| Esophagogastric junction | 31 (32.0) | 6 (22.2) | ||
| Stomach body | 37 (38.1) | 11 (40.7) | ||
| Antrum | 29 (29.9) | 10 (37.0) | ||
| Tumor length D/cm | 1.323 | 0.250 | ||
| ≤5 | 72 (74.2) | 17 (63.0) | ||
| >5 | 25 (25.8) | 10 (37.0) | ||
| Differentiation | 0.473 | 0.491 | ||
| Moderate | 37 (44.6) | 10 (37.0) | ||
| Poor | 46 (55.4) | 17 (63.0) | ||
| Lauren type | 3.248 | 0.197 | ||
| Intestine | 33 (39.8) | 10 (37.0) | ||
| Diffused | 30 (36.1) | 6 (22.2) | ||
| Mixed | 20 (24.1) | 11 (40.7) | ||
| Tumor regression grade | 8.557 | 0.003 | ||
| ≤2 | 66 (68.0) | 10 (37.0) | ||
| 3 | 31 (32.0) | 17 (63.0) | ||
| Nerve invasion | 2.630 | 0.105 | ||
| Yes | 37 (38.1) | 15 (55.6) | ||
| No | 60 (61.9) | 12 (44.4) | ||
| Vascular tumor embolus | 1.563 | 0.211 | ||
| Yes | 18 (18.6) | 8 (29.6) | ||
| No | 79 (81.4) | 19 (70.4) | ||
| ypT | 1.591 | 0.207 | ||
| T1-3 | 34 (35.1) | 6 (22.2) | ||
| T4 | 63 (64.9) | 21 (77.8) | ||
| ypN | 5.509 | 0.019 | ||
| N0 | 46 (47.4) | 6 (22.2) | ||
| N+ | 51 (52.6) | 21 (77.8) | ||
| ypM | 1.151 | 0.576 | ||
| M0 | 93 (95.9) | 27 (100.0) | ||
| M1 | 4 (4.1) | 0 (0.0) | ||
| [1] | International Agency for Research on Cancer World Health Organization IARC. GLOBOCAN 2012: estimated cancer incidence, mortality and prevalence worldwide in 2012[J]. Globocan, 2012, 2012: 3-6. |
| [2] |
STRONG V E, WU A W, SELBY L V, et al. Differences in gastric cancer survival between the US and China[J]. J Surg Oncol, 2015, 112(1): 31-37.
doi: 10.1002/jso.23940 pmid: 26175203 |
| [3] | 赵群, 李勇, 乔喜, 等. 胃癌组织中HER2蛋白的过表达与临床病理的相关分析[J]. 肿瘤学杂志, 2015, 21(5): 360-364. |
| ZHAO Q, LI Y, QIAO X, et al. Correlation between overexpression of HER2 protein and clinicopathology in gastric cancer[J]. J Chin Oncol, 2015, 21(5): 360-364. | |
| [4] |
BANG Y J, VAN CUTSEM E, FEYEREISLOVA A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial[J]. Lancet, 2010, 376(9742): 687-697.
doi: 10.1016/S0140-6736(10)61121-X |
| [5] |
KUROKAWA Y, MATSUURA N, KIMURA Y, et al. Multicenter large-scale study of prognostic impact of HER2 expression in patients with resectable gastric cancer[J]. Gastric Cancer, 2015, 18(4): 691-697.
doi: 10.1007/s10120-014-0430-7 pmid: 25224659 |
| [6] |
YAGI S, WAKATSUKI T, YAMAMOTO N, et al. Clinical significance of intratumoral HER2 heterogeneity on trastuzumab efficacy using endoscopic biopsy specimens in patients with advanced HER2 positive gastric cancer[J]. Gastric Cancer, 2019, 22(3): 518-525.
doi: 10.1007/s10120-018-0887-x pmid: 30328533 |
| [7] |
MOTOSHIMA S, YONEMOTO K, KAMEI H, et al. Prognostic implications of HER2 heterogeneity in gastric cancer[J]. Oncotarget, 2018, 9(10): 9262-9272.
doi: 10.18632/oncotarget.24265 pmid: 29507688 |
| [8] |
LORDICK F, KANG Y K, CHUNG H C, et al. Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): a randomised, open-label phase 3 trial[J]. Lancet Oncol, 2013, 14(6): 490-499.
doi: 10.1016/S1470-2045(13)70102-5 pmid: 23594786 |
| [9] |
QAISER T, RAJPOOT N M. Learning where to see: a novel attention model for automated immunohistochemical scoring[J]. IEEE Trans Med Imaging, 2019, 38(11): 2620-2631.
doi: 10.1109/TMI.42 |
| [10] |
ZHAO Q, LIAN C H, HUO Z B, et al. The efficacy and safety of neoadjuvant chemotherapy on patients with advanced gastric cancer: a multicenter randomized clinical trial[J]. Cancer Med, 2020, 9(16): 5731-5745.
doi: 10.1002/cam4.v9.16 |
| [11] | ZHANG J, TIAN K, DONG P, et al. Microscope based HER2 scoring system[EB/OL]. 2020: arXiv: 2009.06816. https://arxiv.org/abs/2009.06816 [2022-09-01]. |
| [12] | 《胃癌HER2检测指南2016版》专家组. 胃癌HER2检测指南(2016版)[J]. 中华病理学杂志, 2016, 45(8): 528-532. |
| HER2 Detection Guidelines for Gastric Cancer (2016 edition) Expert Group. HER2 detection guidelines for gastric cancer (2016 edition)[J]. Chin J Pathol, 2016, 45(8): 528-532. | |
| [13] |
BARTLEY A N, WASHINGTON M K, COLASACCO C, et al. HER2 testing and clinical decision making in gastroesophageal adenocarcinoma: guideline from the College of American Pathologists, American Society for Clinical Pathology, and the American Society of Clinical Oncology[J]. J Clin Oncol, 2017, 35(4): 446-464.
doi: 10.1200/JCO.2016.69.4836 pmid: 28129524 |
| [14] | JI J, SHEN L, LI Z, et al. Perioperative chemotherapy of oxaliplatin combined with S-1 (SOX) versus postoperative chemotherapy of SOX or oxaliplatin with capecitabine (XELOX) in locally advanced gastric adenocarcinoma with D2 gastrectomy: a randomized phase Ⅲ trial (RESOLVE trial)[J]. Ann Oncol, 2019, 30: v877. |
| [15] | 殷科, 曹永晋. 曲妥珠单抗新辅助化疗表皮生长因子受体2阳性乳腺癌的临床疗效及安全性评价[J]. 中国临床药理学杂志, 2015, 31(9): 725-728. |
| YIN K, CAO Y J. Study on efficacy and safety of trastuzumab chemotherapy for the treatment of human epidermal growth factor receptor-2 positive breast cancer[J]. Chin J Clin Pharmocol, 2015, 31(9): 725-728. | |
| [16] |
CHUA C, TAN I B, YAMADA Y, et al. Phase Ⅱ study of trastuzumab in combination with S-1 and cisplatin in the first-line treatment of human epidermal growth factor receptor HER2-positive advanced gastric cancer[J]. Cancer Chemother Pharmacol, 2015, 76(2): 397-408.
doi: 10.1007/s00280-015-2811-y |
| [17] |
RYU M H, YOO C, KIM J G, et al. Multicenter phase Ⅱ study of trastuzumab in combination with capecitabine and oxaliplatin for advanced gastric cancer[J]. Eur J Cancer, 2015, 51(4): 482-488.
doi: 10.1016/j.ejca.2014.12.015 |
| [18] |
HE Q F, CHEN J H, ZHOU K, et al. Effect of additional trastuzumab in neoadjuvant and adjuvant treatment for patients with resectable HER2-positive gastric cancer[J]. Ann Surg Oncol, 2021, 28(8): 4413-4422.
doi: 10.1245/s10434-020-09405-6 pmid: 33393029 |
| [19] |
YUE M, ZHANG J, WANG X R, et al. Can AI-assisted microscope facilitate breast HER2 interpretation? A multi-institutional ring study[J]. Virchows Arch, 2021, 479(3): 443-449.
doi: 10.1007/s00428-021-03154-x |
| [20] |
TUOMINEN V J, TOLONEN T T, ISOLA J. ImmunoMembrane: a publicly available web application for digital image analysis of HER2 immunohistochemistry[J]. Histopathology, 2012, 60(5): 758-767.
doi: 10.1111/j.1365-2559.2011.04142.x pmid: 22296215 |
| [21] |
HELIN H O, TUOMINEN V J, YLINEN O, et al. Free digital image analysis software helps to resolve equivocal scores in HER2 immunohistochemistry[J]. Virchows Arch, 2016, 468(2): 191-198.
doi: 10.1007/s00428-015-1868-7 |
| [22] |
HEDNER C, BORG D, NODIN B, et al. Expression and prognostic significance of human epidermal growth factor receptors 1, 2 and 3 in oesophageal and gastric adenocarcinomas preneoadjuvant and postneoadjuvant treatment[J]. J Clin Pathol, 2018, 71(5): 451-462.
doi: 10.1136/jclinpath-2017-204774 pmid: 29138285 |
| [23] |
WATSON S, VALIDIRE P, CERVERA P, et al. Combined HER2 analysis of biopsies and surgical specimens to optimize detection of trastuzumab-eligible patients in eso-gastric adenocarcinoma: a GERCOR study[J]. Ann Oncol, 2013, 24(12): 3035-3039.
doi: 10.1093/annonc/mdt393 pmid: 24114855 |
| [24] |
JORDAN N V, BARDIA A, WITTNER B S, et al. HER2 expression identifies dynamic functional states within circulating breast cancer cells[J]. Nature, 2016, 537(7618): 102-106.
doi: 10.1038/nature19328 |
| [25] |
PUSZTAI L, VIALE G, KELLY C M, et al. Estrogen and HER2 receptor discordance between primary breast cancer and metastasis[J]. Oncol, 2010, 15(11): 1164-1168.
doi: 10.1634/theoncologist.2010-0059 |
| [26] |
WETZEL C L, SUTTON T L, GARDINER S, et al. Loss of HER2-positivity following neoadjuvant targeted therapy for breast cancer is not associated with inferior oncologic outcomes[J]. J Surg Oncol, 2021, 124(8): 1224-1234.
doi: 10.1002/jso.26646 pmid: 34416025 |
| [27] |
SEO S, RYU M H, PARK Y S, et al. Loss of HER2 positivity after anti-HER2 chemotherapy in HER2-positive gastric cancer patients: results of the GASTric cancer HER2 reassessment study 3 (GASTHER3)[J]. Gastric Cancer, 2019, 22(3): 527-535.
doi: 10.1007/s10120-018-0891-1 pmid: 30386954 |
| [28] | 于观贞, 魏培莲, 陈颖, 等. 人工智能在肿瘤病理诊断和评估中的应用与思考[J]. 第二军医大学学报, 2017, 38(11): 1349-1354. |
| YU G Z, WEI P L, CHEN Y, et al. Artificial intelligence in pathological diagnosis and assessment of human solid tumor: application and thinking[J]. Acad J Second Mil Med Univ, 2017, 38(11): 1349-1354. |
| [1] | TAN Hong, LIN Shenggeng, XIONG Yi. Current status and challenges of artificial intelligence-enabled prediction of synergistic cancer drug combinations [J]. China Oncology, 2024, 34(9): 807-813. |
| [2] | LIU Shuai, ZHANG Kai, ZHANG Xiaoqing, LUAN Wei. An exploratory study on the perioperative treatment of locally advanced gastric cancer with combination of penpulimab, anlotinib and chemotherapy [J]. China Oncology, 2024, 34(7): 659-668. |
| [3] | WANG Fei, LIU Pei, HU Nan. Effect of bevacizumab assisted PD-1 inhibitor on serum miR-20a-5p and miR-515-3p in the treatment of gastric cancer [J]. China Oncology, 2024, 34(5): 493-500. |
| [4] | Professional Committee on Gastric Cancer of Shanghai Anticancer Association , Professional Committee on Gastrointestinal Cancer of China Association for Promotion of Health Science and Technology . Chinese expert consensus on clinical practice of locally advanced gastric cancer invading adjacent organs (2024 edition) [J]. China Oncology, 2024, 34(5): 517-526. |
| [5] | ZHUANG Han, HU Weigang, ZHANG Zhen, WANG Jiazhou. Deep learning-based lymphocyte infiltration detection on pathological images [J]. China Oncology, 2024, 34(4): 409-417. |
| [6] | XU Yonghu, XU Dazhi. Progress and prospects of gastric cancer treatment in the 21st century [J]. China Oncology, 2024, 34(3): 239-249. |
| [7] | WANG Xuefei, ZHOU Peng, TANG Zhaoqing. New progress and development trend of surgical treatment for gastric cancer [J]. China Oncology, 2024, 34(3): 250-258. |
| [8] | XUE Chi, GAO Peng, ZHU Zhi, WANG Zhenning. Application and challenge of immunotherapy in perioperative therapy of gastric cancer [J]. China Oncology, 2024, 34(3): 259-267. |
| [9] | SHEN Jie, WANG Jiangli, WANG Zezhou, MO Miao, ZHOU Changming, YUAN Jing, XU Dazhi, ZHENG Ying. Survival analysis of 6 737 surgically resected gastric cancer cases in China from a large single institution hospital-based cancer registry database [J]. China Oncology, 2024, 34(3): 268-277. |
| [10] | LI Jing, ZHENG Lei, GAO Yu. Analysis of effects of trastuzumab assisted modified DOF fortnightly regimen on serum tumor markers and survival rate in patients with cisplatin-resistant gastric cancer [J]. China Oncology, 2024, 34(3): 286-292. |
| [11] | JIANG Mengqi, HAN Yuchen, FU Xiaolong. Research progress on H-E stained whole slide image analysis by artificial intelligence in lung cancer [J]. China Oncology, 2024, 34(3): 306-315. |
| [12] | WU Hongji, WANG Haixia, WANG Ling, LUO Xiaogang, ZOU Dongling. Application progress and challenges of artificial intelligence in organoid research [J]. China Oncology, 2024, 34(2): 210-219. |
| [13] | WANG Shanshan, YE Dingwei, YANG Li, CHENG Fan, YANG Tiejun, ZHANG Xiaoping, YU Zhixian, ZHANG Qingyun, YANG Yong. Correlation of HER2 expression and clinicopathological characteristics in patients with urothelial carcinoma in China [J]. China Oncology, 2024, 34(11): 1011-1019. |
| [14] | FENG Huizhi, LIU Jingmei, BU Xiaoqian. A retrospective study of pembrolizumab combined with XELOX regimen in the treatment of advanced gastric cancer [J]. China Oncology, 2024, 34(11): 1028-1035. |
| [15] | ZHAO Junxiu, ZHU Yi, SONG Xiaoyu, ZHE Chao, XIAO Yuhan, LIU Yunduo, LI Linhai, XIAO Bin. Circ-0007766 acts as a miR-1972 sponge to promote breast cancer cell migration and invasion via upregulation of HER2 [J]. China Oncology, 2024, 34(10): 915-930. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
沪ICP备12009617
Powered by Beijing Magtech Co. Ltd
