Welcome to China Oncology,
China Oncology ›› 2023, Vol. 33 ›› Issue (9): 818-828.doi: 10.19401/j.cnki.1007-3639.2023.09.002
• Article • Previous Articles Next Articles
WU Han( ), XU Lei, WANG Miaomiao, ZHANG Ruizhe, XU Xiaoyang, GUO Ningjie, WU Shuhua(
), XU Lei, WANG Miaomiao, ZHANG Ruizhe, XU Xiaoyang, GUO Ningjie, WU Shuhua( )
)
Received:2023-07-18
Revised:2023-09-12
Online:2023-09-30
Published:2023-10-01
Contact:
WU Shuhua.
Share article
CLC Number:
WU Han, XU Lei, WANG Miaomiao, ZHANG Ruizhe, XU Xiaoyang, GUO Ningjie, WU Shuhua. Correlation of LC3 and the recruitment of dendritic cell and the formation of TLS in colorectal cancer and its clinical significance[J]. China Oncology, 2023, 33(9): 818-828.
Tab. 1
RTFQ-PCR primer sequence"
| Gene | Sequence (3’-5’) | |
|---|---|---|
| Forward primer | Reverse primer | |
| LC3 | GGCGCTTACAGCTCAATGCT | CTCCTGGGAGGCATAGACCA |
| NY-ESO-2 | CTTCTGCGCAGGATGGAAG | ATCAACAGGGAAAGCTGCTG |
| CXCL13 | TCTCTGCTTCTCATGCTGCT | TTGTGTAATAGACCTCCAGAACAC |
| LTB | CCAGCTGCCCACCTCATA | GAAACGCCTGTTCCTTCGT |
| CCL21 | GAACCAAGCTTAGGCTGCTC | CTTTGGGTCTGCACATAGCTC |
| IL-17 | TGGGAAGACCTCATTGGTGT | GGATTTCGTGGGATTGTGAT |
| GAPDH | GGCTCTCCAGAACATCATCCCTGC | GGGTGTCGCTGTTGAAGTCAGAGG |
Tab. 2
The expression of LC3 and DC-lamp in colorectal cancer tissues and para-cancerous tissues [n (%)]"
| Item | Case n | LC3 | Pvalue | DC-lamp | P value | ||
|---|---|---|---|---|---|---|---|
| + | - | + | - | ||||
| Para-cancerous tissues | 179 | 25 (13.9) | 154 (86.1) | 0.001 | 48 (26.8) | 131 (73.2) | 0.001 |
| Cancer tissues | 179 | 103 (57.6) | 76 (42.4) | 93 (51.9) | 86 (49.1) | ||
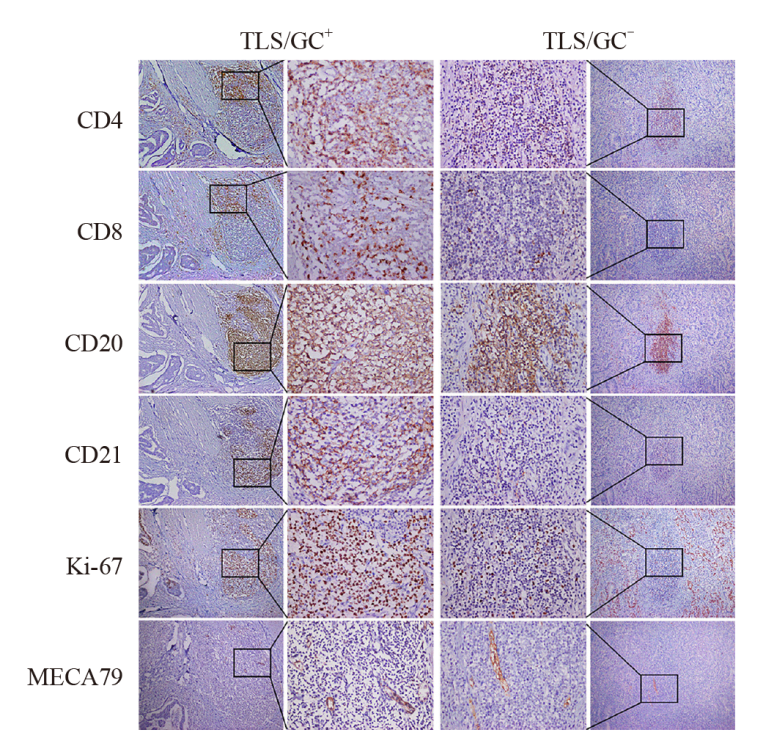
Fig. 2
The expression of marker in TLS/GC+, TLS/GC- subtype in colorectal cancer The left image showed the formation of aggregates of CD4+, CD8+and CD20+ lymphocytes of the TLS/GC+ subtype, with CD21+, Ki-67+ expression around MECA79+; The right image showed the formation of aggregates of CD4+, CD8+and CD20+ lymphocytes of the TLS/GC- subtype, without CD21+, Ki-67+ expression around MECA79+ (in the same field on serial sections of the same sample, immunohistochemical EnVision staining, ×100). Middle boxed area: The same area of marker (immunohistochemical EnVision staining, ×400)."
Tab. 4
The expression and correlation of LC3 at difffferent subgroups in colorectal cancer"
| TLS+ | TLS- | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Item | Case n | GC+ | rvalue | Pvalue | GC- | rvalue | Pvalue | DLI | rvalue | Pvalue | SLI | rvalue | Pvalue | |||||
| + | - | + | - | + | - | + | - | |||||||||||
| LC3 | 0.270 | 0.001 | 0.230 | 0.009 | -0.175 | 0.031 | -0.443 | 0.001 | ||||||||||
| + | 103 | 35 | 86 | 52 | 51 | 13 | 90 | 3 | 100 | |||||||||
| - | 76 | 5 | 71 | 23 | 53 | 20 | 56 | 28 | 48 | |||||||||
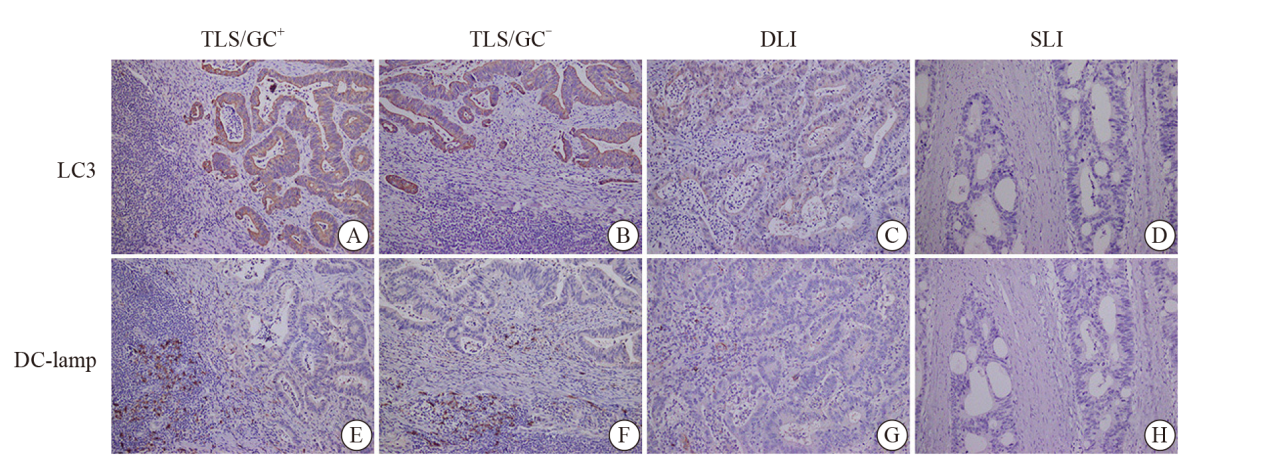
Fig. 3
The expression of LC3 and DC-lamp at different subgroups in colorectal cancer A-D: the expressions of LC3 in different subgroups, which were strongly positive, positive, slightly positive and negative respectively; E-H: the expressions of DC-lamp in different subgroups, which were abundant, relatively abundant, low, and absent in DC-lamp cells (in the same field on serial sections of the same sample, immunohistochemical EnVision staining, ×200)."
Tab. 5
The expression and correlation of DC-lamp in different subgroups in colorectal cancer"
| TLS+ | TLS- | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Item | Case n | GC+ | rvalue | Pvalue | GC- | rvalue | Pvalue | DLI | rvalue | Pvalue | SLI | rvalue | Pvalue | |||||
| + | - | + | - | + | - | + | - | |||||||||||
| DC-lamp | 0.194 | 0.012 | 0.227 | 0.003 | -0.148 | 0.047 | -0.358 | 0.001 | ||||||||||
| + | 93 | 28 | 65 | 49 | 44 | 12 | 81 | 4 | 89 | |||||||||
| - | 86 | 12 | 74 | 26 | 60 | 21 | 65 | 27 | 59 | |||||||||
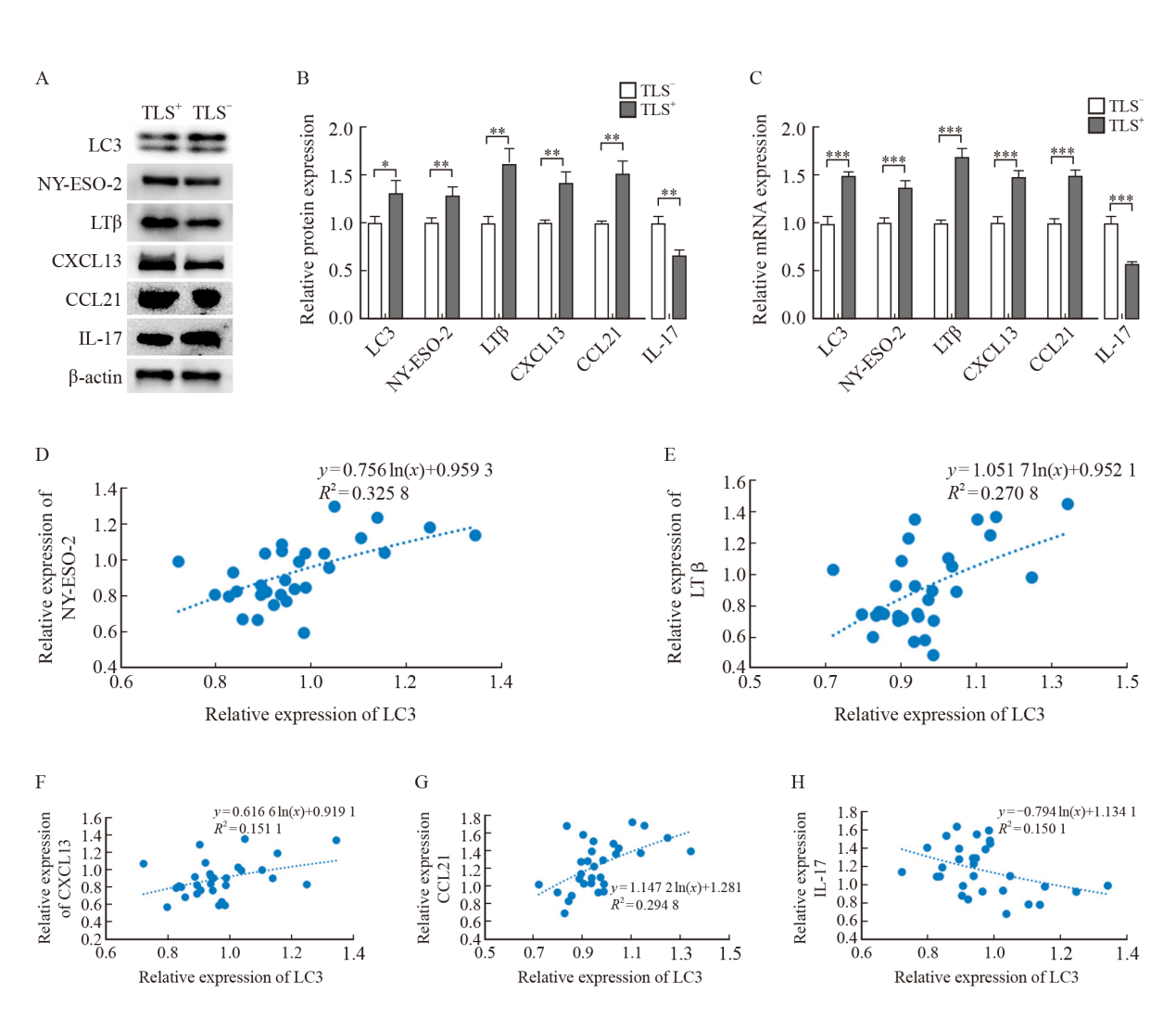
Fig. 4
The expression of LC3, NY-ESO-2, LTβ, CXCL13, CCL21 and IL-17 in TLS+ or TLS- groups and the correlation between LC3 and various factors in colorectal cancer A: Electrophoretic picture; B, C: Relative expression of protein and mRNA; D-H: Analysis of the correlation between LC3 and NY-ESO-2, LTβ, CXCL13, CCL21 and IL-17 in colorectal cancer by logarithmic trend line of linear regression. *: P<0.05, compared with TLS- group; **: P<0.01, compared with TLS- group; ***: P<0.001, compared with TLS- group."
Tab. 6
Multivariable analysis of prognosis in patients with colorectal cancer"
| Item | B | SE | Wald χ2 | df | Exp (B) | 95.0% CI | Pvalue | |
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| LC3 | -0.604 | 0.293 | 4.253 | 1 | 0.547 | 0.308 | 0.970 | 0.039 |
| DC-lamp | -0.993 | 0.288 | 10.463 | 1 | 0.393 | 0.224 | 0.692 | 0.001 |
| TLS | -0.695 | 0.289 | 5.780 | 1 | 0.499 | 0.283 | 0.879 | 0.016 |
| Age | -0.189 | 0.259 | 0.533 | 1 | 0.828 | 0.499 | 1.375 | 0.456 |
| Gender | -0396 | 0.250 | 2.505 | 1 | 0.673 | 0.412 | 1.099 | 0.113 |
| Tumor size | 0.328 | 0.246 | 1.775 | 1 | 1.389 | 0.857 | 2.251 | 0.183 |
| Differentiation | 0.009 | 0.184 | 0.002 | 1 | 1.009 | 0.704 | 1.447 | 0.961 |
| Pathological pattern | 0.158 | 0.319 | 0.246 | 1 | 1.172 | 0.627 | 2.190 | 0.620 |
| Infiltration | -0.114 | 0.303 | 0.142 | 1 | 0.892 | 0.492 | 1.616 | 0.706 |
| Lymph node metastasis | -0.682 | 0.256 | 7.091 | 1 | 0.506 | 0.306 | 0.835 | 0.008 |
| [1] |
SUNG H, FERLAY J, SIEGEL R L, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for36 cancers in 185 countries[J]. CA Cancer J Clin, 2021, 71(3): 209-249.
doi: 10.3322/caac.v71.3 |
| [2] |
SCHUMACHER T N, THOMMEN D S. Tertiary lymphoid structures in cancer[J]. Science, 2022, 375(6576): eabf9419.
doi: 10.1126/science.abf9419 |
| [3] |
DI CARO G, BERGOMAS F, GRIZZI F, et al. Occurrence of tertiary lymphoid tissue is associated with T-cell infiltration and predicts better prognosis in early-stage colorectal cancers[J]. Clin Cancer Res, 2014, 20(8): 2147-2158.
doi: 10.1158/1078-0432.CCR-13-2590 pmid: 24523438 |
| [4] | 范守仁, 吴淑华, 李扬扬, 等. 结直肠癌中LC3与不同表型肿瘤相关巨噬细胞的相关性及其临床意义[J]. 中国癌症杂志, 2020, 30(11): 849-857. |
| [5] | FAN S R, WU S H, LI Y Y, et al. The correlation between LC3 and tumor-associated macrophages in colorectal cancer and its clinical significance[J]. China Oncol, 2020, 30(11): 849-857. |
| [6] | WHO Classification of Tumours Editorial Board. WHO classification of tumours of digestive system[M]. Lyon: IARC Press, 2019. |
| [7] |
TANAKA T, MASUDA A, INOUE J, et al. Integrated analysis of tertiary lymphoid structures in relation to tumor-infiltrating lymphocytes and patient survival in pancreatic ductal adenocarcinoma[J]. J Gastroenterol, 2023, 58(3): 277-291.
doi: 10.1007/s00535-022-01939-8 |
| [8] |
XIA H J, GREEN D R, ZOU W P. Autophagy in tumour immunity and therapy[J]. Nat Rev Cancer, 2021, 21(5): 281-297.
doi: 10.1038/s41568-021-00344-2 pmid: 33758415 |
| [9] |
ZHAO Y, CODOGNO P, ZHANG H. Machinery, regulation and pathophysiological implications of autophagosome maturation[J]. Nat Rev Mol Cell Biol, 2021, 22(11): 733-750.
doi: 10.1038/s41580-021-00392-4 |
| [10] |
MÜNZ C. Canonical and non-canonical functions of the autophagy machinery in MHC restricted antigen presentation[J]. Front Immunol, 2022, 13: 868888.
doi: 10.3389/fimmu.2022.868888 |
| [11] |
ROMAO S, MÜNZ C. LC3-associated phagocytosis[J]. Autophagy, 2014, 10(3): 526-528.
doi: 10.4161/auto.27606 pmid: 24413059 |
| [12] |
JIN Y, SUN C J, FENG L Q, et al. Regulation of SIV antigen-specific CD4+ T cellular immunity via autophagosome-mediated MHC Ⅱ molecule-targeting antigen presentation in mice[J]. PLoS One, 2014, 9(3): e93143.
doi: 10.1371/journal.pone.0093143 |
| [13] | SHANTHA KUMARA H M, GRIECO M J, CABALLERO O L, et al. MAGE-A3 is highly expressed in a subset of colorectal cancer patients[J]. Cancer Immun, 2012, 12: 16. |
| [14] | 刘梦瑶, 吴淑华, 温菲菲, 等. 结直肠癌中LC3与CD4+、CD8+、CD68+免疫细胞浸润的相关性及其临床意义[J]. 临床与实验病理学杂志, 2018, 34(2): 124-131. |
| [15] | LIU M Y, WU S H, WEN F F, et al. Correlation of LC3 and CD4+, CD8+ and CD68+ cell infiltration in colorectal cancer and its clinical significance[J]. Chin J Clin Exp Pathol, 2018, 34(2): 124-131. |
| [16] |
MERAD M, SATHE P, HELFT J, et al. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting[J]. Annu Rev Immunol, 2013, 31: 563-604.
doi: 10.1146/annurev-immunol-020711-074950 pmid: 23516985 |
| [17] |
MILDNER A, JUNG S. Development and function of dendritic cell subsets[J]. Immunity, 2014, 40(5): 642-656.
doi: 10.1016/j.immuni.2014.04.016 pmid: 24837101 |
| [18] |
COLLIN M, BIGLEY V. Human dendritic cell subsets: an update[J]. Immunology, 2018, 154(1): 3-20.
doi: 10.1111/imm.12888 pmid: 29313948 |
| [19] |
FRIDMAN W H, MEYLAN M, PETITPREZ F, et al. B cells and tertiary lymphoid structures as determinants of tumour immune contexture and clinical outcome[J]. Nat Rev Clin Oncol, 2022, 19(7): 441-457.
doi: 10.1038/s41571-022-00619-z pmid: 35365796 |
| [20] |
YAMAMOTO K, VENIDA A, YANO J, et al. Autophagy promotes immune evasion of pancreatic cancer by degrading MHC-I[J]. Nature, 2020, 581(7806): 100-105.
doi: 10.1038/s41586-020-2229-5 |
| [21] |
TIAN Y, KUO C F, SIR D, et al. Autophagy inhibits oxidative stress and tumor suppressors to exert its dual effect on hepatocarcinogenesis[J]. Cell Death Differ, 2015, 22(6): 1025-1034.
doi: 10.1038/cdd.2014.201 pmid: 25526090 |
| [22] |
MAOZ A, DENNIS M, GREENSON J K. The Crohn’s-like lymphoid reaction to colorectal cancer-tertiary lymphoid structures with immunologic and potentially therapeutic relevance in colorectal cancer[J]. Front Immunol, 2019, 10: 1884.
doi: 10.3389/fimmu.2019.01884 |
| [23] |
SAUTÈS-FRIDMAN C, PETITPREZ F, CALDERARO J, et al. Tertiary lymphoid structures in the era of cancer immunotherapy[J]. Nat Rev Cancer, 2019, 19(6): 307-325.
doi: 10.1038/s41568-019-0144-6 |
| [24] |
WANG B, LIU J, HAN Y, et al. The presence of tertiary lymphoid structures provides new insight into the clinicopathological features and prognosis of patients with breast cancer[J]. Front Immunol, 2022, 13: 868155.
doi: 10.3389/fimmu.2022.868155 |
| [25] |
SILIŅA K, SOLTERMANN A, ATTAR F M, et al. Germinal centers determine the prognostic relevance of tertiary lymphoid structures and are impaired by corticosteroids in lung squamous cell carcinoma[J]. Cancer Res, 2018, 78(5): 1308-1320.
doi: 10.1158/0008-5472.CAN-17-1987 pmid: 29279354 |
| [26] |
DING G Y, MA J Q, YUN J P, et al. Distribution and density of tertiary lymphoid structures predict clinical outcome in intrahepatic cholangiocarcinoma[J]. J Hepatol, 2022, 76(3): 608-618.
doi: 10.1016/j.jhep.2021.10.030 |
| [27] |
WEINSTEIN A M, CHEN L, BRZANA E A, et al. Tbet and IL-36γ cooperate in therapeutic DC-mediated promotion of ectopic lymphoid organogenesis in the tumor microenvironment[J]. OncoImmunology, 2017, 6(6): e1322238.
doi: 10.1080/2162402X.2017.1322238 |
| [28] |
HALLE S, DUJARDIN H C, BAKOCEVIC N, et al. Induced bronchus-associated lymphoid tissue serves as a general priming site for T cells and is maintained by dendritic cells[J]. J Exp Med, 2009, 206(12): 2593-2601.
doi: 10.1084/jem.20091472 |
| [29] |
COLBECK E J, AGER A, GALLIMORE A, et al. Tertiary lymphoid structures in cancer: drivers of antitumor immunity, immunosuppression, or bystander sentinels in disease?[J]. Front Immunol, 2017, 8: 1830.
doi: 10.3389/fimmu.2017.01830 pmid: 29312327 |
| [30] |
J GUNDERSON A, RAJAMANICKAM V, BUI C, et al. Germinal center reactions in tertiary lymphoid structures associate with neoantigen burden, humoral immunity and long-term survivorship in pancreatic cancer[J]. Oncoimmunology, 2021, 10(1): 1900635.
doi: 10.1080/2162402X.2021.1900635 |
| [31] |
WANG Q Y, SHEN X F, AN R, et al. Peritumoral tertiary lymphoid structure and tumor stroma percentage predict the prognosis of patients with non-metastatic colorectal cancer[J]. Front Immunol, 2022, 13: 962056.
doi: 10.3389/fimmu.2022.962056 |
| [32] |
LI S, LIN Z, ZHENG W, et al. IL-17A inhibits autophagic activity of HCC cells by inhibiting the degradation of Bcl2[J]. Biochem Biophys Res Commun, 2019, 509(1): 194-200.
doi: 10.1016/j.bbrc.2018.12.103 |
| [33] |
DIEU-NOSJEAN M C, GIRALDO N A, KAPLON H, et al. Tertiary lymphoid structures, drivers of the anti-tumor responses in human cancers[J]. Immunol Rev, 2016, 271(1): 260-275.
doi: 10.1111/imr.2016.271.issue-1 |
| [34] |
CALDERARO J, PETITPREZ F, BECHT E, et al. Intra-tumoral tertiary lymphoid structures are associated with a low risk of early recurrence of hepatocellular carcinoma[J]. J Hepatol, 2019, 70(1): 58-65.
doi: S0168-8278(18)32373-0 pmid: 30213589 |
| [35] |
ALLEMANI C, MATSUDA T, DI CARLO V, et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries[J]. Lancet, 2018, 391(10125): 1023-1075.
doi: 10.1016/S0140-6736(17)33326-3 |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
沪ICP备12009617
Powered by Beijing Magtech Co. Ltd
