Welcome to China Oncology,
China Oncology ›› 2024, Vol. 34 ›› Issue (1): 13-66.doi: 10.19401/j.cnki.1007-3639.2024.01.002
• Specialist' Commentary • Previous Articles Next Articles
Colorectal Cancer Special Committee of Shanghai Anti-Cancer Association
Received:2023-11-24
Revised:2023-12-06
Online:2024-01-30
Published:2024-02-05
Share article
CLC Number:
Colorectal Cancer Special Committee of Shanghai Anti-Cancer Association . Shanghai plan for early screening, diagnosis and treatment of colorectal cancer (2023 edition)[J]. China Oncology, 2024, 34(1): 13-66.
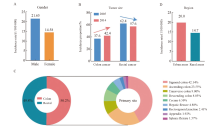
Fig. 2
Characteristics of colorectal cancer incidence in China A: There are obvious gender differences in the incidence of colorectal cancer in China; B: From 2005 to 2014, the proportion of colon cancer patients in China continued to increase, while the proportion of rectal cancer patients gradually decreased; C: An overview of the incidence of colon cancer sub-sites further broken down according to the anatomical parts of the colon; D: The incidence of colorectal cancer in China shows obvious differences in urban and rural distribution."
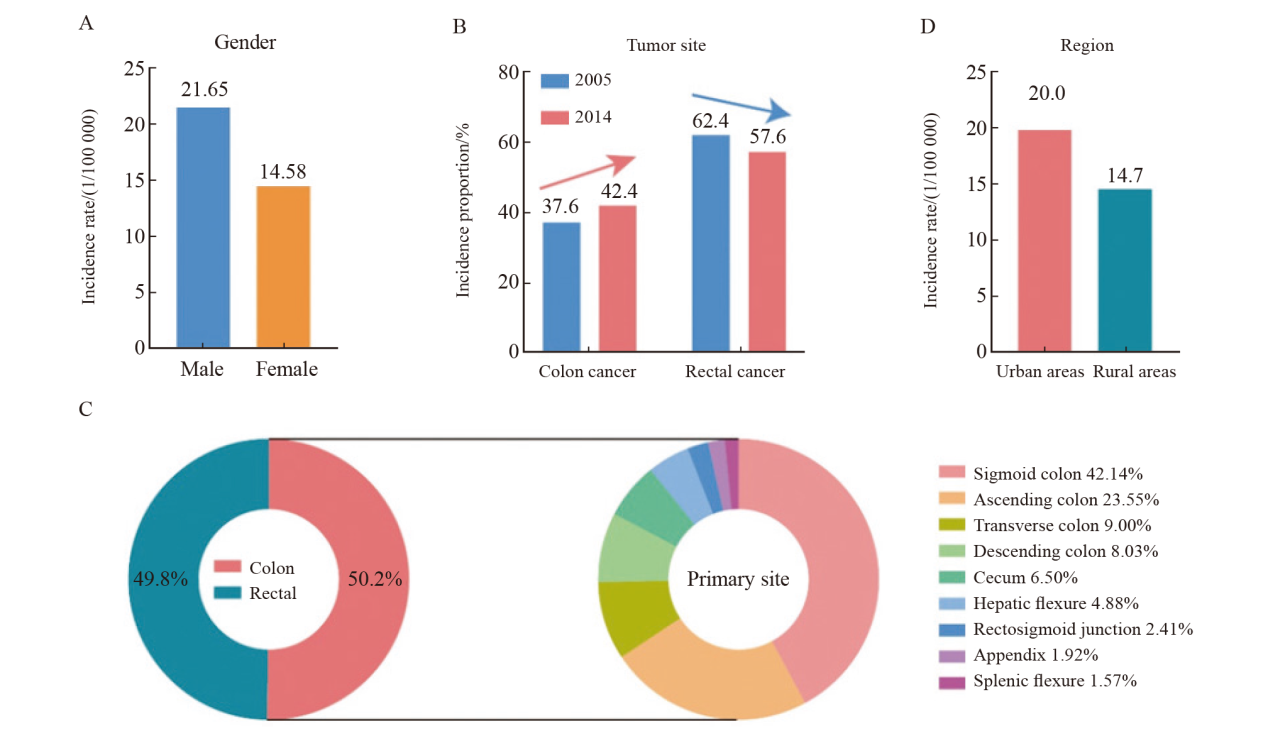
Fig. 3
Colorectal cancer mortality trends in China A: Overview of top 10 cancers in terms of new deaths reported in China in 2016; B: Colorectal cancer mortality among men and women in China; C: China’s colorectal cancer mortality rate shows obvious differences in distribution between urban and rural areas; D: Proportion of colorectal cancer patients receiving surgical treatment alone in China. OS: Overall survival."
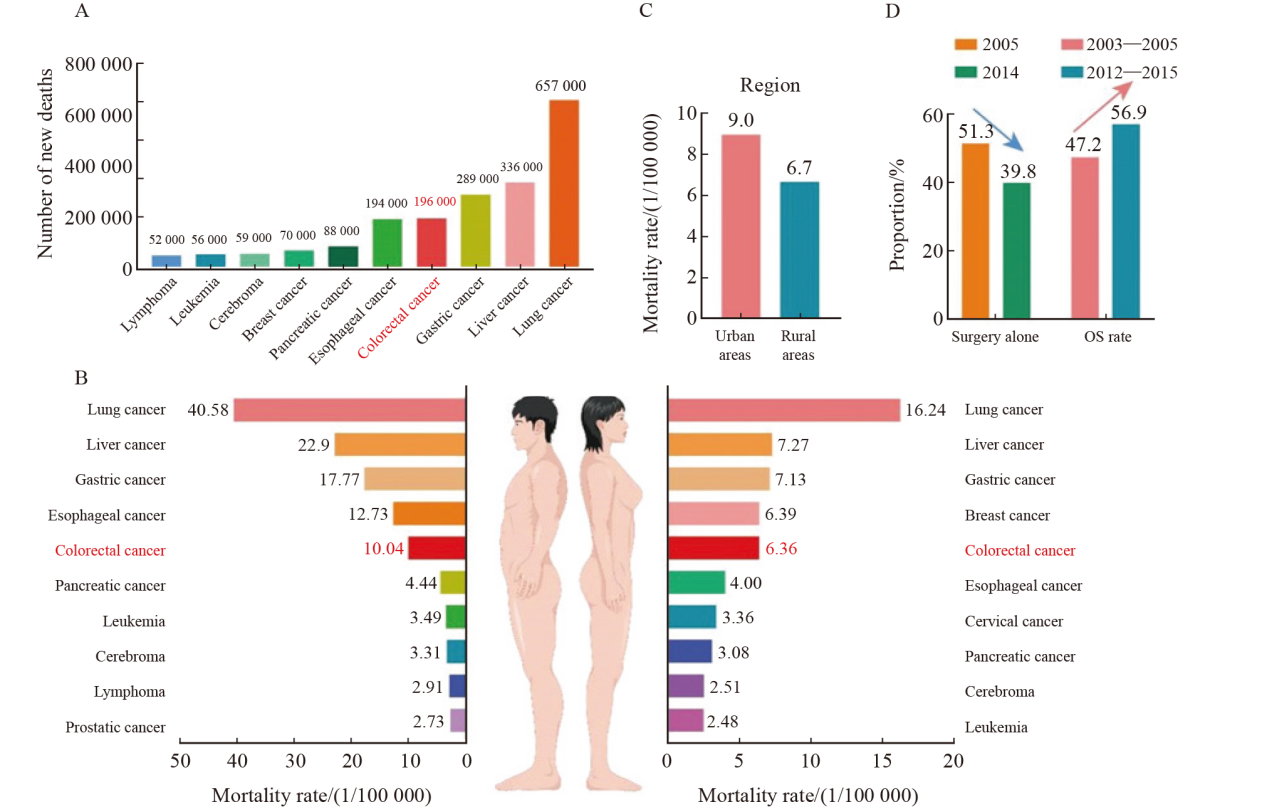
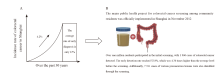
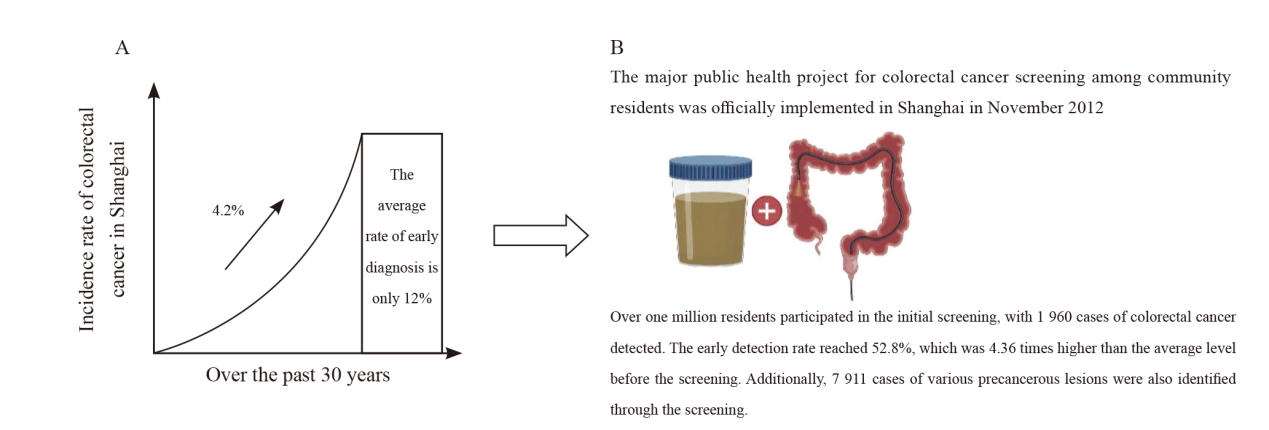
Fig. 4
Colorectal cancer incidence situation in Shanghai A: As a typical representative of China’s urban areas and eastern regions, Shanghai has the most significant growth rate of colorectal cancer incidence; B: In November 2012, a major public health project in Shanghai-the colorectal cancer screening project for community residents was officially launched."
Tab. 1
List of hereditary colorectal cancers and colorectal cancer risks"
| Hereditary colorectal cancer | Main mutated gene | Inheritance | Disease characteristic | Recommended age to start screening | Screening interval |
|---|---|---|---|---|---|
| LS | MMR, EPCAM | Autosomal dominant inheritance | The most common, in addition to colorectal cancer, is also associated with malignant tumors in multiple systems such as endometrial cancer, ovarian cancer, gastric cancer, liver cancer, pancreatic cancer, and renal pelvis cancer | 20 to 25 years old, or 2 to 5 years earlier than the age of onset of the youngest patient in the family | Once every 1 to 2 years, once a year after the age of 40 |
| FAP | APC | Autosomal dominant inheritance | A large number of colonic adenomatous polyps. If left untreated, the risk of developing colorectal cancer is close to 100%. Extracolonic manifestations are often present | Typical FAP: Start colonoscopy screening at 10 to 11 years old; Mild FAP: Start at 18 to 20 years old | Typical FAP: Colonoscopy is performed every 1 to 2 years and lasts for life; Mild FAP: Colonoscopy is performed every 2 years and lasts for life |
| MAP | MUTYH | Autosomal recessive inheritance | Multiple colorectal polyps with extracolonic manifestations | Age 40 or 10 years earlier than the age at which a first-degree relative is diagnosed with colorectal cancer | Once every 1 to 2 years, once a year after the age of 40 |
| FCCX | Uncertain | Uncertain | Large heterogeneity, less extracolonic manifestations | 5 to 10 years earlier than the earliest age of diagnosis in the family | Once every 3 to 5 years |
Tab. 2
The AJCC 8th edition TNM staging system"
| TNM staging | T | N | M |
|---|---|---|---|
| 0 | In situ | 0 | 0 |
| Ⅰ | 1 | 0 | 0 |
| 2 | 0 | 0 | |
| ⅡA | 3 | 0 | 0 |
| ⅡB | 4a | 0 | 0 |
| ⅡC | 4b | 0 | 0 |
| ⅢA | 1 | 1a | 0 |
| 1 | 1b | 0 | |
| 1 | 1c | 0 | |
| 2 | 1a | 0 | |
| 2 | 1b | 0 | |
| 2 | 1c | 0 | |
| 1 | 2a | 0 | |
| ⅢB | 1 | 2b | 0 |
| 2 | 2a | 0 | |
| 2 | 2b | 0 | |
| 3 | 1a | 0 | |
| 3 | 1b | 0 | |
| 3 | 1c | 0 | |
| 3 | 2a | 0 | |
| 4a | 1a | 0 | |
| 4a | 1b | 0 | |
| 4a | 1c | 0 | |
| ⅢC | 3 | 2b | 0 |
| 4a | 2a | 0 | |
| 4a | 2b | 0 | |
| 4b | 1a | 0 | |
| 4b | 1b | 0 | |
| 4b | 1c | 0 | |
| 4b | 2a | 0 | |
| 4b | 2b | 0 | |
| ⅣA | Any T | Any N | 1a |
| ⅣB | Any T | Any N | 1b |
| ⅣC | Any T | Any N | 1c |
Tab. 3
Characteristics of consensus molecular subtypes of colorectal cancer"
| Item | CMS1 | CMS2 | CMS3 | CMS4 |
|---|---|---|---|---|
| MSI | MSI-H | MSS | MSS | MSS |
| CpG islamd methylation phenotype | High | — | Low | — |
| Somatic abundance | Normal | High | Low | High |
| Mutation | BRAF | — | KRAS | — |
| Immune infiltration | High | Low | Low | Normal |
| Stromal infiltration | Normal | Low | Low | High |
| Epithelial cells/mesenchymal cells | — | Epithelial cells | Epithelial cells | Mesenchymal cells |
| Signal channel | High JAK/STAT | High Wnt | High metabolic | High TGF-β/VEGF |
| Survival state | Good prognosis | - | - | Poor prognosis |
Tab. 4
The colorectal cancer screening population risk stratification scoring system"
| Risk factor | APCS score | APCS score (revised edition) | Colorectal tumor prediction score | |||||
|---|---|---|---|---|---|---|---|---|
| Standard | Score | Standard | Score | Standard | Score | |||
| Age/year | <50 | 0 | 40-49 | 0 | 50-55 | 0 | ||
| 50-69 | 2 | 50-59 | 1 | 56-70 | 1 | |||
| ≥70 | 3 | ≥60 | 2 | |||||
| Gender | Female | 0 | Female | 0 | Female | 0 | ||
| Male | 1 | Male | 1 | Male | 1 | |||
| Family history of colorectal cancer in a first-degree relative | No | 0 | No | 0 | No | 0 | ||
| Yes | 2 | Yes | 1 | Yes | 1 | |||
| Smoking | Never | 0 | Never | 0 | Never | 0 | ||
| Current/past | 1 | Current/past | 1 | Current/past | 1 | |||
| BMI | <23 kg/m2 | 0 | <25 kg/m2 | 0 | ||||
| ≥23 kg/m2 | 1 | ≥25 kg/m2 | 1 | |||||
| Self-reported diabetes | No | 0 | ||||||
| Yes | 1 | |||||||
| Risk level | Low risk | 0-1 | Low risk | 0 | Low risk | 0-2 | ||
| Moderate risk | 2-3 | Moderate risk | 1-3 | High risk | 3-6 | |||
| High risk | 4-7 | High risk | 4-6 | |||||
| Risk prediction | Colorectal cancer and advanced adenoma risk | Colorectal cancer and advanced adenoma risk | Colorectal adenomas, advanced adenomas and overall risk of colorectal cancer | |||||
Tab. 5
Comparing the advantages and disadvantages of stool-based examination methods"
| Inspection mean | Advantage | Disadvantage |
|---|---|---|
| FOBT | Low cost and easy to operate | ① Lower sensitivity; ② Easily affected by diet; ③ High false positive rate |
| FIT test | ① Fewer dietary restrictions than FOBT; ② Higher specificity in diagnosis | ① Poorer sensitivity in detecting precancerous lesions; ② Results might be interfered with by certain foods and medications |
| Multi-target stool DNA test | ① Greater sensitivity in diagnosis; ② Significantly higher sensitivity in detecting precancerous lesions; ③ Hardly any dietary restrictions | High cost which may hinder its promotion in large-scale population screening |
Tab. 6
Comparison of screening strategies for the general population at home and abroad"
| Country or region | Initial screenig | Final screening | Screening method and frequency |
|---|---|---|---|
| America[ | 45 | 75 | Variety of ways |
| Japan[ | 40 | FIT once a year | |
| Australia[ | 50 | 74 | FIT once every two years |
| Canada[ | 50 | 74 | FIT or high-sensitivity gFOBT once every two years |
| China[ | 40 | 75 | FIT once a year or colonoscopy every 5 to 10 years |
| Shanghai[ | 45 | 75 | FIT once a year |
| [1] | ZHENG R S, ZHANG S W, ZENG H M, et al. Cancer incidence and mortality in China, 2016[J]. J Natl Cancer Cent, 2022, 2(1): 1-9. |
| [2] |
SUNG H, FERLAY J, SIEGEL R L, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2021, 71(3): 209-249.
doi: 10.3322/caac.v71.3 |
| [3] |
SHI J F, WANG L, RAN J C, et al. Clinical characteristics, medical service utilization, and expenditure for colorectal cancer in China, 2005 to 2014: overall design and results from a multicenter retrospective epidemiologic survey[J]. Cancer, 2021, 127(11): 1880-1893.
doi: 10.1002/cncr.v127.11 |
| [4] | 王锡山. 从中美结直肠癌流行病学特征看结直肠癌早诊早治的重要性[J]. 中华结直肠疾病电子杂志, 2021, 10(1): 26-33. |
| WANG X S. Discussion of the importance of early diagnosis and treatment of colorectal cancer from the epidemiological characteristics of colorectal cancer in China and United States of America[J]. Chin J Colorectal Dis Electron Ed, 2021, 10(1): 26-33. | |
| [5] |
CHEN W Q, ZHENG R S, BAADE P D, et al. Cancer statistics in China, 2015[J]. CA Cancer J Clin, 2016, 66(2): 115-132.
doi: 10.3322/caac.v66.2 |
| [6] | ZHANG S W, SUN K X, ZHENG R S, et al. Cancer incidence and mortality in China, 2015[J]. J Natl Cancer Cent, 2021, 1(1): 2-11. |
| [7] | WAN D S. Epidemiologic trend of and strategies for colorectal cancer[J]. Ai Zheng, 2009, 28(9): 897-902. |
| [8] |
GONG Y, PENG P, BAO P, et al. The implementation and first-round results of a community-based colorectal cancer screening program in Shanghai, China[J]. Oncologist, 2018, 23(8): 928-935.
doi: 10.1634/theoncologist.2017-0451 pmid: 29540604 |
| [9] | 吴春晓, 龚杨明, 顾凯, 等. 2016年上海市结肠直肠癌发病和死亡情况与2002—2016年间的变化趋势分析[J]. 外科理论与实践, 2021, 26(4): 325-335. |
| WU C X, GONG Y M, GU K, et al. Colorectal cancer incidence and mortality in Shanghai 2016 and trend analysis 2002-2016[J]. J Surg Concepts Pract, 2021, 26(4): 325-335. | |
| [10] | 郑莹, 龚杨明. 上海地区人群大肠癌筛查的研究和实践[J]. 中国肿瘤, 2013, 22(2): 86-89. |
| ZHENG Y, GONG Y M. Research and practice of screening for colorectal cancer in population of Shanghai[J]. China Cancer, 2013, 22(2): 86-89. | |
| [11] | 龚杨明, 顾凯, 彭鹏, 等. 社区居民大肠癌筛查工作规范解读[J]. 上海预防医学, 2017, 29(2): 99-101. |
| GONG Y M, GU K, PENG P, et al. Interpretation of the norms of colorectal cancer screening for community residents[J]. Shanghai J Prev Med, 2017, 29(2): 99-101. | |
| [12] |
KASTRINOS F, SAMADDER N J, BURT R W. Use of family history and genetic testing to determine risk of colorectal cancer[J]. Gastroenterology, 2020, 158(2): 389-403.
doi: S0016-5085(19)41585-0 pmid: 31759928 |
| [13] |
LOWERY J T, AHNEN D J, SCHROY P C Ⅲ, et al. Understanding the contribution of family history to colorectal cancer risk and its clinical implications: a state-of-the-science review[J]. Cancer, 2016, 122(17): 2633-2645.
doi: 10.1002/cncr.30080 pmid: 27258162 |
| [14] |
SAMADDER N J, CURTIN K, TUOHY T M, et al. Increased risk of colorectal neoplasia among family members of patients with colorectal cancer: a population-based study in Utah[J]. Gastroenterology, 2014, 147(4): 814-821.e5; quize15.
doi: 10.1053/j.gastro.2014.07.006 pmid: 25042087 |
| [15] |
WIN A K, JENKINS M A, DOWTY J G, et al. Prevalence and penetrance of major genes and polygenes for colorectal cancer[J]. Cancer Epidemiol Biomarkers Prev, 2017, 26(3): 404-412.
doi: 10.1158/1055-9965.EPI-16-0693 |
| [16] |
UMAR A, TERDIMAN J P, et al. Revised Bethesda guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability[J]. J Natl Cancer Inst, 2004, 96(4): 261-268.
doi: 10.1093/jnci/djh034 pmid: 14970275 |
| [17] |
MA X Y, ZHANG B, ZHENG W. Genetic variants associated with colorectal cancer risk: comprehensive research synopsis, meta-analysis, and epidemiological evidence[J]. Gut, 2014, 63(2): 326-336.
doi: 10.1136/gutjnl-2012-304121 pmid: 23946381 |
| [18] |
LINDOR N M, RABE K, PETERSEN G M, et al. Lower cancer incidence in Amsterdam-Ⅰ criteria families without mismatch repair deficiency: familial colorectal cancer type Ⅹ[J]. JAMA, 2005, 293(16): 1979-1985.
doi: 10.1001/jama.293.16.1979 |
| [19] | ZETNER D B, BISGAARD M L. Familial colorectal cancer type X[J]. Curr Genom, 2017, 18(4): 341-359. |
| [20] |
YAMAGUCHI T, FURUKAWA Y, NAKAMURA Y, et al. Comparison of clinical features between suspected familial colorectal cancer type Ⅹ and Lynch syndrome in Japanese patients with colorectal cancer: a cross-sectional study conducted by the Japanese Society for Cancer of the Colon and Rectum[J]. Jpn J Clin Oncol, 2015, 45(2): 153-159.
doi: 10.1093/jjco/hyu190 |
| [21] |
SÁNCHEZ-TOMÉ E, RIVERA B, PEREA J, et al. Genome-wide linkage analysis and tumoral characterization reveal heterogeneity in familial colorectal cancer type Ⅹ[J]. J Gastroenterol, 2015, 50(6): 657-666.
doi: 10.1007/s00535-014-1009-0 |
| [22] |
SEHGAL R, SHEAHAN K, O'CONNELL P R, et al. Lynch syndrome: an updated review[J]. Genes, 2014, 5(3): 497-507.
doi: 10.3390/genes5030497 pmid: 24978665 |
| [23] | MOUSAVI S M, FALLAH M, SUNDQUIST K, et al. Age- and time-dependent changes in cancer incidence among immigrants to Sweden: colorectal, lung, breast and prostate cancers[J]. Int J Cancer, 2012, 131(2): E122-E128. |
| [24] |
REZENDE L F M, SÁ T H, MARKOZANNES G, et al. Physical activity and cancer: an umbrella review of the literature including 22 major anatomical sites and 770 000 cancer cases[J]. Br J Sports Med, 2018, 52(13): 826-833.
doi: 10.1136/bjsports-2017-098391 |
| [25] |
RUIZ-CASADO A, MARTÍN-RUIZ A, PÉREZ L M, et al. Exercise and the hallmarks of cancer[J]. Trends Cancer, 2017, 3(6): 423-441.
doi: 10.1016/j.trecan.2017.04.007 |
| [26] |
MA P, YAO Y, SUN W, et al. Daily sedentary time and its association with risk for colorectal cancer in adults: a dose-response meta-analysis of prospective cohort studies[J]. Medicine (Baltimore), 2017, 96(22): e7049.
doi: 10.1097/MD.0000000000007049 |
| [27] |
LYNCH B M. Sedentary behavior and cancer: a systematic review of the literature and proposed biological mechanisms[J]. Cancer Epidemiol Biomarkers Prev, 2010, 19(11): 2691-2709.
doi: 10.1158/1055-9965.EPI-10-0815 |
| [28] |
KIKUCHI N, NISHIYAMA T, SAWADA T, et al. Perceived stress and colorectal cancer incidence: the Japan collaborative cohort study[J]. Sci Rep, 2017, 7: 40363.
doi: 10.1038/srep40363 pmid: 28091607 |
| [29] |
ZHANG Q, BERGER F G, LOVE B, et al. Maternal stress and early-onset colorectal cancer[J]. Med Hypotheses, 2018, 121: 152-159.
doi: S0306-9877(18)30615-7 pmid: 30396471 |
| [30] |
TILG H, ADOLPH T E, GERNER R R, et al. The intestinal microbiota in colorectal cancer[J]. Cancer Cell, 2018, 33(6): 954-964.
doi: S1535-6108(18)30072-2 pmid: 29657127 |
| [31] |
HOLOWATYJ A N, PEREA J, LIEU C H. Gut instinct: a call to study the biology of early-onset colorectal cancer disparities[J]. Nat Rev Cancer, 2021, 21(6): 339-340.
doi: 10.1038/s41568-021-00356-y pmid: 33833408 |
| [32] |
CESPEDES E M, HU F B. Dietary patterns: from nutritional epidemiologic analysis to national guidelines[J]. Am J Clin Nutr, 2015, 101(5): 899-900.
doi: 10.3945/ajcn.115.110213 pmid: 25832336 |
| [33] |
TABUNG F K, BROWN L S, FUNG T T. Dietary patterns and colorectal cancer risk: a review of 17 years of evidence (2000-2016)[J]. Curr Colorectal Cancer Rep, 2017, 13(6): 440-454.
doi: 10.1007/s11888-017-0390-5 pmid: 29399003 |
| [34] |
VIEIRA A R, ABAR L, CHAN D S M, et al. Foods and beverages and colorectal cancer risk: a systematic review and meta-analysis of cohort studies, an update of the evidence of the WCRF-AICR Continuous Update Project[J]. Ann Oncol, 2017, 28(8): 1788-1802.
doi: 10.1093/annonc/mdx171 pmid: 28407090 |
| [35] |
HUR J, OTEGBEYE E, JOH H K, et al. Sugar-sweetened beverage intake in adulthood and adolescence and risk of early-onset colorectal cancer among women[J]. Gut, 2021, 70(12): 2330-2336.
doi: 10.1136/gutjnl-2020-323450 pmid: 33958435 |
| [36] |
CHOI Y J, MYUNG S K, LEE J H. Light alcohol drinking and risk of cancer: a meta-analysis of cohort studies[J]. Cancer Res Treat, 2018, 50(2): 474-487.
doi: 10.4143/crt.2017.094 |
| [37] |
GIOVANNUCCI E. Alcohol, one-carbon metabolism, and colorectal cancer: recent insights from molecular studies[J]. J Nutr, 2004, 134(9): 2475S-2481S.
pmid: 15333745 |
| [38] |
GIOVANNUCCI E, ELENA MARTINEZ M. Tobacco, colorectal cancer, and adenomas: a review of the evidence[J]. J Natl Cancer Inst, 1996, 88(23): 1717-1730.
pmid: 8944002 |
| [39] |
LIANG P S, CHEN T Y, GIOVANNUCCI E. Cigarette smoking and colorectal cancer incidence and mortality: systematic review and meta-analysis[J]. Int J Cancer, 2009, 124(10): 2406-2415.
doi: 10.1002/ijc.24191 pmid: 19142968 |
| [40] |
RENEHAN A G, TYSON M, EGGER M, et al. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies[J]. Lancet, 2008, 371(9612): 569-578.
doi: 10.1016/S0140-6736(08)60269-X pmid: 18280327 |
| [41] |
SONG M Y, HU F B, SPIEGELMAN D, et al. Long-term status and change of body fat distribution, and risk of colorectal cancer: a prospective cohort study[J]. Int J Epidemiol, 2016, 45(3): 871-883.
doi: 10.1093/ije/dyv177 pmid: 26403814 |
| [42] |
LI H, BOAKYE D, CHEN X, et al. Association of body mass index with risk of early-onset colorectal cancer: systematic review and meta-analysis[J]. Am J Gastroenterol, 2021, 116(11): 2173-2183.
doi: 10.14309/ajg.0000000000001393 pmid: 34309586 |
| [43] |
LIU P H, WU K, NG K, et al. Association of obesity with risk of early-onset colorectal cancer among women[J]. JAMA Oncol, 2019, 5(1): 37-44.
doi: 10.1001/jamaoncol.2018.4280 |
| [44] |
LIM U, ERNST T, BUCHTHAL S D, et al. Asian women have greater abdominal and visceral adiposity than Caucasian women with similar body mass index[J]. Nutr Diabetes, 2011, 1(5): e6.
doi: 10.1038/nutd.2011.2 |
| [45] |
KIM H, GIOVANNUCCI E L. Sex differences in the association of obesity and colorectal cancer risk[J]. Cancer Causes Control, 2017, 28(1): 1-4.
doi: 10.1007/s10552-016-0831-5 |
| [46] |
KIM J Y, JUNG Y S, PARK J H, et al. Different risk factors for advanced colorectal neoplasm in young adults[J]. World J Gastroenterol, 2016, 22(13): 3611-3620.
doi: 10.3748/wjg.v22.i13.3611 |
| [47] |
SAETANG J, SANGKHATHAT S. Diets link metabolic syndrome and colorectal cancer development[J]. Oncol Rep, 2017, 37(3): 1312-1320.
doi: 10.3892/or.2017.5385 |
| [48] |
DREWES J L, CHEN J, MARKHAM N O, et al. Human colon cancer-derived clostridioides difficile strains drive colonic tumorigenesis in mice[J]. Cancer Discov, 2022, 12(8): 1873-1885.
doi: 10.1158/2159-8290.CD-21-1273 pmid: 35678528 |
| [49] |
SONG M, CHAN A T, SUN J. Influence of the gut microbiome, diet, and environment on risk of colorectal cancer[J]. Gastroenterology, 2020, 158(2): 322-340.
doi: S0016-5085(19)41369-3 pmid: 31586566 |
| [50] |
YANG Y, DU L, SHI D, et al. Dysbiosis of human gut microbiome in young-onset colorectal cancer[J]. Nat Commun, 2021, 12(1): 6757.
doi: 10.1038/s41467-021-27112-y pmid: 34799562 |
| [51] |
SIMIN J, FORNES R, LIU Q, et al. Antibiotic use and risk of colorectal cancer: a systematic review and dose-response meta-analysis[J]. Br J Cancer, 2020, 123(12): 1825-1832.
doi: 10.1038/s41416-020-01082-2 |
| [52] |
CAO Y, WU K, MEHTA R, et al. Long-term use of antibiotics and risk of colorectal adenoma[J]. Gut, 2018, 67(4): 672-678.
doi: 10.1136/gutjnl-2016-313413 pmid: 28377387 |
| [53] |
ZHANG J, SEARS C L. Antibiotic use impacts colorectal cancer: a double-edged sword by tumor location?[J]. J Natl Cancer Inst, 2022, 114(1): 1-2.
doi: 10.1093/jnci/djab126 |
| [54] | 蔡三军, 赵任. 大肠癌: 基础与临床的转化[M]. 上海: 上海交通大学出版社, 2020. |
| CAI S J, ZHAO R. Colorectal cancer: transformation from basic to clinical practice[M]. Shanghai: Shanghai Jiao Tong University Press, 2020. | |
| [55] |
HAMID H K S. Schistosoma japonicum-associated colorectal cancer: a review[J]. Am J Trop Med Hyg, 2019, 100(3): 501-505.
doi: 10.4269/ajtmh.18-0807 |
| [56] |
HOLSCHER H D. Dietary fiber and prebiotics and the gastrointestinal microbiota[J]. Gut Microbes, 2017, 8(2): 172-184.
doi: 10.1080/19490976.2017.1290756 pmid: 28165863 |
| [57] |
ZENG H W, TAUSSIG D P, CHENG W H, et al. Butyrate inhibits cancerous HCT116 colon cell proliferation but to a lesser extent in noncancerous NCM460 colon cells[J]. Nutrients, 2017, 9(1): 25.
doi: 10.3390/nu9010025 |
| [58] |
ELCE A, AMATO F, ZARRILLI F, et al. Butyrate modulating effects on pro-inflammatory pathways in human intestinal epithelial cells[J]. Benef Microbes, 2017, 8(5): 841-847.
doi: 10.3920/BM2016.0197 pmid: 28856908 |
| [59] |
PARTULA V, DESCHASAUX M, DRUESNE-PECOLLO N, et al. Associations between consumption of dietary fibers and the risk of cardiovascular diseases, cancers, type 2 diabetes, and mortality in the prospective NutriNet-Santé cohort[J]. Am J Clin Nutr, 2020, 112(1): 195-207.
doi: 10.1093/ajcn/nqaa063 pmid: 32369545 |
| [60] |
KOUSHIK A, HUNTER D J, SPIEGELMAN D, et al. Fruits, vegetables, and colon cancer risk in a pooled analysis of 14 cohort studies[J]. J Natl Cancer Inst, 2007, 99(19): 1471-1483.
pmid: 17895473 |
| [61] |
LEE J E, CHAN A T. Fruit, vegetables, and folate: cultivating the evidence for cancer prevention[J]. Gastroenterology, 2011, 141(1): 16-20.
doi: 10.1053/j.gastro.2011.05.020 pmid: 21620843 |
| [62] |
SHAUKAT A, SCOURAS N, SCHÜNEMANN H J. Role of supplemental calcium in the recurrence of colorectal adenomas: a meta analysis of randomized controlled trials[J]. Am J Gastroenterol, 2005, 100(2): 390-394.
doi: 10.1111/ajg.2005.100.issue-2 |
| [63] |
BOYLE T, KEEGEL T, BULL F, et al. Physical activity and risks of proximal and distal colon cancers: a systematic review and meta-analysis[J]. J Natl Cancer Inst, 2012, 104(20): 1548-1561.
doi: 10.1093/jnci/djs354 pmid: 22914790 |
| [64] |
MCTIERNAN A, FRIEDENREICH C M, KATZMARZYK P T, et al. Physical activity in cancer prevention and survival: a systematic review[J]. Med Sci Sports Exerc, 2019, 51(6): 1252-1261.
doi: 10.1249/MSS.0000000000001937 |
| [65] |
GRANCHER A, MICHEL P, DI FIORE F, et al. Aspirine et cancer colorectal[J]. Bull Du Cancer, 2018, 105(2): 171-180.
doi: 10.1016/j.bulcan.2017.09.013 |
| [66] |
ROTHWELL P M, WILSON M, ELWIN C E, et al. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials[J]. Lancet, 2010, 376(9754): 1741-1750.
doi: 10.1016/S0140-6736(10)61543-7 pmid: 20970847 |
| [67] |
DITONNO I, LOSURDO G, RENDINA M, et al. Estrogen receptors in colorectal cancer: facts, novelties and perspectives[J]. Curr Oncol, 2021, 28(6): 4256-4263.
doi: 10.3390/curroncol28060361 pmid: 34898546 |
| [68] |
GRAY R T, COLEMAN H G, HUGHES C, et al. Statin use and survival in colorectal cancer: results from a population-based cohort study and an updated systematic review and meta-analysis[J]. Cancer Epidemiol, 2016, 45: 71-81.
doi: S1877-7821(16)30191-6 pmid: 27750068 |
| [69] | CHENG Z, LIU Z. Renin-angiotensin system gene polymorphisms and colorectal cancer risk: a meta-analysis[J]. J Renin Angiotensin Aldosterone Syst, 2019, 20(4): 1470320319881932. |
| [70] |
JACOBS E T, VAN PELT C, FORSTER R E, et al. CYP24A1 and CYP27B1 polymorphisms modulate vitamin D metabolism in colon cancer cells[J]. Cancer Res, 2013, 73(8): 2563-2573.
doi: 10.1158/0008-5472.CAN-12-4134 pmid: 23423976 |
| [71] | 国家消化系统疾病临床医学研究中心上海, 中华医学会消化内镜学分会, 中国抗癌协会肿瘤内镜专业委员会, 等. 中国结直肠癌癌前病变和癌前状态处理策略专家共识[J]. 中华消化内镜杂志, 2022, 39(1): 1-18. |
| National Clinical Research Center for Digestive Diseases Shanghai, Chinese Society of Digestive Endoscopology, Cancer Endoscopy Professional Committee of China Anti-Cancer Association. Expert consensus on management strategies for precancerous lesions and conditions of colorectal cancer in China[J]. Chin J Dig Endosc, 2022, 39(1): 1-18. | |
| [72] | WHO Classification of Tumours Editorial Board. WHO classification of tumours of digestive system[M]. Lyon: IARC Press, 2019. |
| [73] | 国家消化系统疾病临床医学研究中心上海, 中华医学会消化内镜学分会, 中国抗癌协会肿瘤内镜专业委员会, 等. 中国结直肠癌癌前病变和癌前状态处理策略专家共识[J]. 中华消化内镜杂志, 2022, 39(1): 1-18. |
| National Clinical Research Center for Digestive Diseases Shanghai, Chinese Society of Digestive Endoscopology, Cancer Endoscopy Professional Committee of China Anti-Cancer Association, et al. Expert consensus on management strategies for precancerous lesions and conditions of colorectal cancer in China[J]. Chin J Dig Endosc, 2022, 39(1): 1-18. | |
| [74] |
SANO Y, TANAKA S, KUDO S E, et al. Narrow-band imaging (NBI) magnifying endoscopic classification of colorectal tumors proposed by the Japan NBI Expert Team[J]. Dig Endosc, 2016, 28(5): 526-533.
doi: 10.1111/den.12644 pmid: 26927367 |
| [75] | PATRUN J, OKREŠA L, IVEKOVIČ H, et al. Diagnostic accuracy of nice classification system for optical recognition of predictive morphology of colorectal polyps[J]. Gastroenterol Res Pract, 2018, 2018: 7531368. |
| [76] |
MINODA Y, OGINO H, CHINEN T, et al. Objective validity of the Japan Narrow-Band Imaging Expert Team classification system for the differential diagnosis of colorectal polyps[J]. Dig Endosc, 2019, 31(5): 544-551.
doi: 10.1111/den.13393 pmid: 30861599 |
| [77] |
BENSON A B, VENOOK A P, AL-HAWARY M M, et al. Colon cancer, version 2.2021, NCCN clinical practice guidelines in oncology[J]. J Natl Compr Canc Netw, 2021, 19(3): 329-359.
doi: 10.6004/jnccn.2021.0012 |
| [78] |
GUINNEY J, DIENSTMANN R, WANG X, et al. The consensus molecular subtypes of colorectal cancer[J]. Nat Med, 2015, 21(11): 1350-1356.
doi: 10.1038/nm.3967 pmid: 26457759 |
| [79] |
JI Y, LV J, SUN D, et al. Therapeutic strategies targeting Wnt/β-catenin signaling for colorectal cancer (review)[J]. Int J Mol Med, 2022, 49(1): 1.
doi: 10.3892/ijmm |
| [80] |
BRYJA V, ČERVENKA I, ČAJÁNEK L. The connections of Wnt pathway components with cell cycle and centrosome: side effects or a hidden logic?[J]. Crit Rev Biochem Mol Biol, 2017, 52(6): 614-637.
doi: 10.1080/10409238.2017.1350135 |
| [81] |
LEE R M, LI J X, LI J, et al. Synthetic essentiality of tryptophan 2, 3-dioxygenase 2 in APC-mutated colorectal cancer[J]. Cancer Discov, 2022, 12(7): 1702-1717.
doi: 10.1158/2159-8290.CD-21-0680 |
| [82] |
ZHANG X X, YAO J N, SHI H L, et al. Hsa_circ_0026628 promotes the development of colorectal cancer by targeting SP1 to activate the Wnt/β-catenin pathway[J]. Cell Death Dis, 2021, 12(9): 802.
doi: 10.1038/s41419-021-03794-6 pmid: 34420031 |
| [83] |
LIU R, DENG P, ZHANG Y, et al. Circ_0082182 promotes oncogenesis and metastasis of colorectal cancer in vitro and in vivo by sponging miR-411 and miR-1205 to activate the Wnt/β-catenin pathway[J]. World J Surg Oncol, 2021, 19(1): 51.
doi: 10.1186/s12957-021-02164-y pmid: 33596920 |
| [84] |
FLANAGAN D J, PENTINMIKKO N, LUOPAJÄRVI K, et al. NOTUM from APC-mutant cells biases clonal competition to initiate cancer[J]. Nature, 2021, 594(7863): 430-435.
doi: 10.1038/s41586-021-03525-z |
| [85] |
VAN NEERVEN S M, DE GROOT N E, NIJMAN L E, et al. APC-mutant cells act as supercompetitors in intestinal tumour initiation[J]. Nature, 2021, 594(7863): 436-441.
doi: 10.1038/s41586-021-03558-4 |
| [86] |
EKSTRAND A I, JÖNSSON M, LINDBLOM A, et al. Frequent alterations of the PI3K/AKT/mTOR pathways in hereditary nonpolyposis colorectal cancer[J]. Fam Cancer, 2010, 9(2): 125-129.
doi: 10.1007/s10689-009-9293-1 pmid: 19731079 |
| [87] |
ENGELMAN J A. Targeting PI3K signalling in cancer: opportunities, challenges and limitations[J]. Nat Rev Cancer, 2009, 9(8): 550-562.
doi: 10.1038/nrc2664 pmid: 19629070 |
| [88] |
STAHLER A, HEINEMANN V, RICARD I, et al. Current treatment options in RAS mutant metastatic colorectal cancer patients: a meta-analysis of 14 randomized phase Ⅲ trials[J]. J Cancer Res Clin Oncol, 2020, 146(8): 2077-2087.
doi: 10.1007/s00432-020-03290-y |
| [89] |
LI F, ZHOU Y D, LIU J, et al. RBP-J promotes cell growth and metastasis through regulating miR-182-5p-mediated Tiam1/Rac1/p38 MAPK axis in colorectal cancer[J]. Cell Signal, 2021, 87: 110103.
doi: 10.1016/j.cellsig.2021.110103 |
| [90] | LI C F, DING D Y, GAO Y J, et al. MicroRNA-3651 promotes colorectal cancer cell proliferation through directly repressing T-box transcription factor 1[J]. Int J Mol Med, 2020, 45(3): 956-966. |
| [91] |
STRAMUCCI L, PRANTEDA A, STRAVATO A, et al. MKK3 sustains cell proliferation and survival through p38DELTA MAPK activation in colorectal cancer[J]. Cell Death Dis, 2019, 10(11): 842.
doi: 10.1038/s41419-019-2083-2 pmid: 31695024 |
| [92] |
ATKINSON C J, KAWAMATA F, LIU C, et al. EGFR and Prion protein promote signaling via FOXO3a-KLF5 resulting in clinical resistance to platinum agents in colorectal cancer[J]. Mol Oncol, 2019, 13(4): 725-737.
doi: 10.1002/1878-0261.12411 pmid: 30478887 |
| [93] |
GEORGIOU A, STEWART A, CUNNINGHAM D, et al. Inactivation of NF1 promotes resistance to EGFR inhibition in KRAS/NRAS/BRAF V600-wild-type colorectal cancer[J]. Mol Cancer Res, 2020, 18(6): 835-846.
doi: 10.1158/1541-7786.MCR-19-1201 |
| [94] |
ELEZ E, ROS J, FERNÁNDEZ J, et al. RNF43 mutations predict response to anti-BRAF/EGFR combinatory therapies in BRAF V600E metastatic colorectal cancer[J]. Nat Med, 2022, 28(10): 2162-2170.
doi: 10.1038/s41591-022-01976-z |
| [95] |
SAKAI D, TANIGUCHI H, SUGIMOTO N, et al. Randomised phase Ⅱ study of panitumumab plus irinotecan versus cetuximab plus irinotecan in patients with KRAS wild-type metastatic colorectal cancer refractory to fluoropyrimidine, irinotecan and oxaliplatin (WJOG 6510G)[J]. Eur J Cancer, 2020, 135: 11-21.
doi: 10.1016/j.ejca.2020.04.014 |
| [96] |
BENNOUNA J, HIRET S, BERTAUT A, et al. Continuation of bevacizumab vs cetuximab plus chemotherapy after first progression in KRAS wild-type metastatic colorectal cancer: the UNICANCER PRODIGE18 randomized clinical trial[J]. JAMA Oncol, 2019, 5(1): 83-90.
doi: 10.1001/jamaoncol.2018.4465 |
| [97] |
CORCORAN R B, ANDRÉ T, ATREYA C E, et al. Combined BRAF, EGFR, and MEK inhibition in patients with BRAF V600E-mutant colorectal cancer[J]. Cancer Discov, 2018, 8(4): 428-443.
doi: 10.1158/2159-8290.CD-17-1226 |
| [98] |
CHEN D, HUANG J F, LIU K, et al. BRAF V600E mutation and its association with clinicopathological features of colorectal cancer: a systematic review and meta-analysis[J]. PLoS One, 2014, 9: e90607.
doi: 10.1371/journal.pone.0090607 |
| [99] |
BRAY F, FERLAY J, SOERJOMATARAM I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2018, 68(6): 394-424.
doi: 10.3322/caac.v68.6 |
| [100] |
ZHANG L, CAO F, ZHANG G, et al. Trends in and predictions of colorectal cancer incidence and mortality in China from 1990 to 2025[J]. Front Oncol, 2019, 9: 98.
doi: 10.3389/fonc.2019.00098 pmid: 30847304 |
| [101] | XIA C F, DONG X S, LI H, et al. Cancer statistics in China and United States, 2022: profiles, trends, and determinants[J]. Chin Med J (Engl), 2022, 135(5): 584-590. |
| [102] |
DE MARTEL C, GEORGES D, BRAY F, et al. Global burden of cancer attributable to infections in 2018: a worldwide incidence analysis[J]. Lancet Glob Health, 2020, 8(2): e180-e190.
doi: 10.1016/S2214-109X(19)30488-7 pmid: 31862245 |
| [103] |
ALLEMANI C, MATSUDA T, DI CARLO V, et al. Global surveillance of trends in cancer survival 2000-2014 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries[J]. Lancet, 2018, 391(10125): 1023-1075.
doi: 10.1016/S0140-6736(17)33326-3 |
| [104] |
SANKARANARAYANAN R, SWAMINATHAN R, BRENNER H, et al. Cancer survival in Africa, Asia, and Central America: a population-based study[J]. Lancet Oncol, 2010, 11(2): 165-173.
doi: 10.1016/S1470-2045(09)70335-3 pmid: 20005175 |
| [105] | 国家消化系统疾病临床医学研究中心上海, 国家消化道早癌防治中心联盟, 中华医学会消化内镜学分会, 等. 中国早期结直肠癌筛查流程专家共识意见(2019,上海)[J]. 中华消化内镜杂志, 2019, 36(10): 709-719. |
| National Clinical Research Center for Digestive Diseases Shanghai, National Early Gastrointestinal-Cancer Prevention & Treatment Center Alliance GECA, Chinese Society of Digestive Endoscopy, et al. Chinese consensus of early colorectal cancer screening (2019, Shanghai)[J]. Chin J Dig Endosc, 2019, 36(10): 709-719. | |
| [106] | 国家癌症中心中国结直肠癌筛查与早诊早治指南制定专家组. 中国结直肠癌筛查与早诊早治指南(2020,北京)[J]. 中华肿瘤杂志, 2021, 43(1): 16-38. |
| National Cancer Center, China, Expert Group of the Development of China Guideline for the Screening, Early Detection and Early Treatment of Colorectal Cancer. China guideline for the screening, early detection and early treatment of colorectal cancer (2020, Beijing)[J]. Chin J Oncol, 2021, 43(1): 16-38. | |
| [107] | 中华医学会肿瘤学分会早诊早治学组. 中国结直肠癌早诊早治专家共识[J]. 中华医学杂志, 2020, 100(22): 1691-1698. |
| Early Diagnosis and Treatment Group of the Oncology Branch of Chinese Medical Association. Expert consensus on early diagnosis and treatment of colorectal cancer in China[J]. Natl Med J China, 2020, 100(22): 1691-1698. | |
| [108] |
BRENNER H, TAO S. Superior diagnostic performance of faecal immunochemical tests for haemoglobin in a head-to-head comparison with guaiac based faecal occult blood test among 2235 participants of screening colonoscopy[J]. Eur J Cancer, 2013, 49(14): 3049-3054.
doi: 10.1016/j.ejca.2013.04.023 pmid: 23706981 |
| [109] |
IMPERIALE T F, RANSOHOFF D F, ITZKOWITZ S H, et al. Multitarget stool DNA testing for colorectal-cancer screening[J]. N Engl J Med, 2014, 370(14): 1287-1297.
doi: 10.1056/NEJMoa1311194 |
| [110] |
MO S B, WANG H, HAN L Y, et al. Fecal multidimensional assay for non-invasive detection of colorectal cancer: fecal immunochemical test, stool DNA mutation, methylation, and intestinal bacteria analysis[J]. Front Oncol, 2021, 11: 643136.
doi: 10.3389/fonc.2021.643136 |
| [111] | 潘辉, 黄琰璎, 王国新, 等. 粪便基因SDC2和BMP3甲基化联合检测在结直肠癌筛查中的价值[J]. 中国医药导报, 2020, 17(11): 15-19. |
| PAN H, HUANG Y Y, WANG G X, et al. Value of combined detection of fecal genes SDC2 and BMP3 methylation in screening for colorectal cancer[J]. Chin Med Herald, 2020, 17(11): 15-19. | |
| [112] |
TSAI W S, YOU J F, HUNG H Y, et al. Novel circulating tumor cell assay for detection of colorectal adenomas and cancer[J]. Clin Transl Gastroenterol, 2019, 10(10): e00088.
doi: 10.14309/ctg.0000000000000088 |
| [113] |
JIN P, KANG Q, WANG X, et al. Performance of a second-generation methylated SEPT9 test in detecting colorectal neoplasm[J]. J Gastroenterol Hepatol, 2015, 30(5): 830-833.
doi: 10.1111/jgh.2015.30.issue-5 |
| [114] |
CAI G X, CAI M Y, FENG Z Q, et al. A multilocus blood-based assay targeting circulating tumor DNA methylation enables early detection and early relapse prediction of colorectal cancer[J]. Gastroenterology, 2021, 161(6): 2053-2056.e2.
doi: 10.1053/j.gastro.2021.08.054 |
| [115] |
MO S, YE L, WANG D, et al. Early detection of molecular residual disease and risk stratification for stage Ⅰ to Ⅲ colorectal cancer via circulating tumor DNA methylation[J]. JAMA Oncol, 2023, 9(6): 770-778.
doi: 10.1001/jamaoncol.2023.0425 |
| [116] |
MO S, DAI W, WANG H, et al. Early detection and prognosis prediction for colorectal cancer by circulating tumour DNA methylation haplotypes: a multicentre cohort study[J]. EClinicalMedicine, 2023, 55: 101717.
doi: 10.1016/j.eclinm.2022.101717 |
| [117] |
MA X J, CHEN Y K, TANG W, et al. Multi-dimensional fragmentomic assay for ultrasensitive early detection of colorectal advanced adenoma and adenocarcinoma[J]. J Hematol Oncol, 2021, 14(1): 175.
doi: 10.1186/s13045-021-01189-w |
| [118] |
ATKIN W, WOOLDRAGE K, PARKIN D M, et al. Long term effects of once-only flexible sigmoidoscopy screening after 17 years of follow-up: the UK flexible sigmoidoscopy screening randomised controlled trial[J]. Lancet, 2017, 389(10076): 1299-1311.
doi: S0140-6736(17)30396-3 pmid: 28236467 |
| [119] |
BAI Y, GAO J, ZOU D W, et al. Distribution trends of colorectal adenoma and cancer: a colonoscopy database analysis of 11 025 Chinese patients[J]. J Gastroenterol Hepatol, 2010, 25(10): 1668-1673.
doi: 10.1111/jgh.2010.25.issue-10 |
| [120] |
VAN GOSSUM A, MUNOZ-NAVAS M, FERNANDEZ-URIEN I, et al. Capsule endoscopy versus colonoscopy for the detection of polyps and cancer[J]. N Engl J Med, 2009, 361(3): 264-270.
doi: 10.1056/NEJMoa0806347 |
| [121] |
SPADA C, PASHA S F, GROSS S A, et al. Accuracy of first- and second-generation colon capsules in endoscopic detection of colorectal polyps: a systematic review and meta-analysis[J]. Clin Gastroenterol Hepatol, 2016, 14(11): 1533-1543.e8.
doi: 10.1016/j.cgh.2016.04.038 |
| [122] |
STJEPANOVIC N, MOREIRA L, CARNEIRO F, et al. Hereditary gastrointestinal cancers: ESMO clinical practice guidelines for diagnosis, treatment and follow-up[J]. Ann Oncol, 2019, 30(10): 1558-1571.
doi: S0923-7534(19)60977-4 pmid: 31987447 |
| [123] | WEISS J M, GUPTA S, BURKE C A, et al. NCCN guidelines® insights: genetic/familial high-risk assessment: colorectal, version 1.2021[J]. J Natl Compr Canc Netw, 2021, 19(10): 1122-1132. |
| [124] |
US PREVENTIVE SERVICES TASK FORCE, DAVIDSON K W, BARRY M J, et al. Screening for colorectal cancer: US preventive services task force recommendation statement[J]. JAMA, 2021, 325(19): 1965-1977.
doi: 10.1001/jama.2021.6238 pmid: 34003218 |
| [125] | National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology, colonrectal cancer screening, version 1. 2023[EB/OL]. [2023-11-25]. https://www.nccn.org/professionals/physician_gls/pdf/colorectal_screening.pdf. |
| [126] |
SAITO Y, OKA S, KAWAMURA T, et al. Colonoscopy screening and surveillance guidelines[J]. Dig Endosc, 2021, 33(4): 486-519.
doi: 10.1111/den.13972 pmid: 33713493 |
| [127] | YOUNG G, Cancer Council Australia Colorectal Cancer Guidelines Working Party. Clinical practice guidelines for the prevention, early detection and management of colorectal cancer[EB/OL]. (2018-01-01)[2023-11-25]. https://wiki.cancer.org.au/australia/guidelines:colorectal_cancer. |
| [128] |
Canadian Task Force on Preventive Health Care. Recommendations on screening for colorectal cancer in primary care[J]. Can Med Assoc J, 2016, 188(5): 340-348.
doi: 10.1503/cmaj.151125 |
| [129] | 上海市抗癌协会. 居民常见恶性肿瘤筛查和预防推荐(2023版)[J]. 抗癌, 2023(2): 1-24. |
| Shanghai Anti-Cancer Association. Recommendation for screening and prevention of common malignant tumors among residents (2023 edition)[J]. Anti-cancer, 2023(2): 1-24. | |
| [130] |
BISSCHOPS R, EAST J E, HASSAN C, et al. Advanced imaging for detection and differentiation of colorectal neoplasia: European Society of Gastrointestinal Endoscopy (ESGE) guideline-update 2019[J]. Endoscopy, 2019, 51(12): 1155-1179.
doi: 10.1055/a-1031-7657 |
| [131] | 刘肖肖, 朱春平, 李兆申. 消化内镜技术在消化道黏膜病变中的应用和进展[J]. 中华消化内镜杂志, 2019, 36(5): 376-380. |
| LIU X X, ZHU C P, LI Z S. Application and progress of digestive endoscopy in gastrointestinal mucosal lesions[J]. Chin J Dig Endosc, 2019, 36(5): 376-380. | |
| [132] |
ROELANDT P, DEMEDTS I, WILLEKENS H, et al. Impact of endoscopy system, high definition, and virtual chromoendoscopy in daily routine colonoscopy: a randomized trial[J]. Endoscopy, 2019, 51(3): 237-243.
doi: 10.1055/a-0755-7471 pmid: 30646403 |
| [133] |
PIOCHE M, DENIS A, ALLESCHER H D, et al. Impact of 2 generational improvements in colonoscopes on adenoma miss rates: results of a prospective randomized multicenter tandem study[J]. Gastrointest Endosc, 2018, 88(1): 107-116.
doi: S0016-5107(18)30055-5 pmid: 29410020 |
| [134] | 李娜, 金鹏, 康倩, 等. 早期结直肠癌白光内镜下特点分析[J]. 胃肠病学和肝病学杂志, 2016, 25(5): 520-523. |
| LI N, JIN P, KANG Q, et al. The characteristics of early colorectal cancer under white light endoscopy[J]. Chin J Gastroenterol Hepatol, 2016, 25(5): 520-523. | |
| [135] |
SHINOZAKI S, KOBAYASHI Y, HAYASHI Y, et al. Colon polyp detection using linked color imaging compared to white light imaging: systematic review and meta-analysis[J]. Dig Endosc, 2020, 32(6): 874-881.
doi: 10.1111/den.v32.6 |
| [136] |
OBA S, TANAKA S, OKA S, et al. Characterization of colorectal tumors using narrow-band imaging magnification: combined diagnosis with both pit pattern and microvessel features[J]. Scand J Gastroenterol, 2010, 45(9): 1084-1092.
doi: 10.3109/00365521003734166 |
| [137] |
TAJIRI H, NIWA H. Proposal for a consensus terminology in endoscopy: how should different endoscopic imaging techniques be grouped and defined?[J]. Endoscopy, 2008, 40(9): 775-778.
doi: 10.1055/s-2008-1077507 pmid: 18698532 |
| [138] |
YANG D H, PARK S J, KIM H S, et al. High-definition chromoendoscopy versus high-definition white light colonoscopy for neoplasia surveillance in ulcerative colitis: a randomized controlled trial[J]. Am J Gastroenterol, 2019, 114(10): 1642-1648.
doi: 10.14309/ajg.0000000000000341 |
| [139] |
KAWAGUTI F S, FRANCO M C, MARTINS B C, et al. Role of magnification chromoendoscopy in the management of colorectal neoplastic lesions suspicious for submucosal invasion[J]. Dis Colon Rectum, 2019, 62(4): 422-428.
doi: 10.1097/DCR.0000000000001343 pmid: 30730457 |
| [140] | 汪望月, 叶洁桐, 叶国良. 双重染色内镜应用于消化道早癌的诊断价值[J]. 中华全科医学, 2018, 16(5): 768-770. |
| WANG W Y, YE J T, YE G L. Diagnostic value of double staining endoscopy in early digestive tract cancer[J]. Chin J Gen Pract, 2018, 16(5): 768-770. | |
| [141] | 蔡世伦, 钟芸诗. 放大内镜窄带成像技术在结直肠早癌诊断中的应用进展[J]. 中华结直肠疾病电子杂志, 2014, 3(6): 12-15. |
| CAI S L, ZHONG Y S. Magnifying endoscopy with narrow-banding imaging: new progress in diagnosis of early stage colorectal cancers[J]. Chin J Colorectal Dis (Electron Ed), 2014, 3(6): 12-15. | |
| [142] |
PUIG I, LÓPEZ-CERÓN M, ARNAU A, et al. Accuracy of the narrow-band imaging international colorectal endoscopic classification system in identification of deep invasion in colorectal polyps[J]. Gastroenterology, 2019, 156(1): 75-87.
doi: S0016-5085(18)35094-7 pmid: 30296432 |
| [143] | 戈之铮, 姜智敏, 萧树东, 等. 自体荧光内镜对消化道肿瘤的诊断价值[J]. 胃肠病学, 2010, 15(5): 267-270. |
| GE Z Z, JIANG Z M, XIAO S D, et al. Diagnostic value of autofluorescence endoscopy in gastrointestinal neoplasms[J]. Chin J Gastroenterol, 2010, 15(5): 267-270. | |
| [144] | 周春燕, 陈群. 自体荧光内镜对消化道肿瘤诊断临床应用分析[J]. 中国现代医师, 2020, 58(2): 13-15. |
| ZHOU C Y, CHEN Q. Clinical application of autofluorescence endoscopy in diagnosis of digestive tract tumors[J]. China Mod Dr, 2020, 58(2):13-15. | |
| [145] |
YOSHIDA N, HISABE T, INADA Y, et al. The ability of a novel blue laser imaging system for the diagnosis of invasion depth of colorectal neoplasms[J]. J Gastroenterol, 2014, 49(1): 73-80.
doi: 10.1007/s00535-013-0772-7 pmid: 23494646 |
| [146] | 刁文秀, 沈磊. 蓝激光内镜结合JNET分型对早期结直肠癌及癌前病变的诊断价值[J]. 中华结直肠疾病电子杂志, 2019, 8(5): 461-468. |
| DIAO W X, SHEN L. The diagnostic ability of blue laser imaging combined with JNET classification for early colorectal cancer and precancerous lesions[J]. Chin J Colorectal Dis, 2019, 8(5): 461-468. | |
| [147] |
COMMITTEE A T. Confocal laser endomicroscopy[J]. Gastrointest Endosc, 2014, 80(6): 928-938.
pmid: 25442092 |
| [148] |
KOULAOUZIDIS A. Technology status evaluation report on wireless capsule endoscopy[J]. Gastrointest Endosc, 2014, 79(5): 872-873.
doi: 10.1016/j.gie.2013.12.033 pmid: 24721630 |
| [149] |
ELIAKIM R, FIREMAN Z, GRALNEK I M, et al. Evaluation of the PillCam Colon capsule in the detection of colonic pathology: results of the first multicenter, prospective, comparative study[J]. Endoscopy, 2006, 38(10): 963-970.
pmid: 17058158 |
| [150] |
PECERE S, SENORE C, HASSAN C, et al. Accuracy of colon capsule endoscopy for advanced neoplasia[J]. Gastrointest Endosc, 2020, 91(2): 406-414.e1.
doi: S0016-5107(19)32336-3 pmid: 31629719 |
| [151] | SAITO H, MACHII R, TAKAHASHI N, et al. Evidence on colorectal cancer screening and a perspective on future research[J]. Nihon Shokakibyo Gakkai Zasshi, 2014, 111(3): 453-463. |
| [152] |
BEYNON J, FOY D M, ROE A M, et al. Endoluminal ultrasound in the assessment of local invasion in rectal cancer[J]. Br J Surg, 1986, 73(6): 474-477.
doi: 10.1002/bjs.1800730618 |
| [153] | 周平红, 姚礼庆, 徐美东, 等. 内镜超声在结直肠癌术前分期中的应用价值[J]. 中华胃肠外科杂志, 2001, 4(4): 237-240. |
| ZHOU P H, YAO L Q, XU M D, et al. Value of endoscopic ultrasonography in preoperative staging of colorectal carcinoma[J]. Chin J Gastrointest Surg, 2001, 4(4): 237-240. | |
| [154] |
SAVIDES T J, MASTER S S. EUS in rectal cancer[J]. Gastrointest Endosc, 2002, 56(4 Suppl): S12-S18.
doi: 10.1016/s0016-5107(02)70079-5 pmid: 12297742 |
| [155] |
GAO Y, LI J, MA X, et al. The value of four imaging modalities in diagnosing lymph node involvement in rectal cancer: an overview and adjusted indirect comparison[J]. Clin Exp Med, 2019, 19(2): 225-234.
doi: 10.1007/s10238-019-00552-z pmid: 30900099 |
| [156] | LI X T, SUN Y S, TANG L, et al. Evaluating local lymph node metastasis with magnetic resonance imaging, endoluminal ultrasound and computed tomography in rectal cancer: a meta-analysis[J]. Colorectal Dis, 2015, 17(6): O129-O135. |
| [157] |
LI X T, ZHANG X Y, SUN Y S, et al. Evaluating rectal tumor staging with magnetic resonance imaging, computed tomography, and endoluminal ultrasound: a meta-analysis[J]. Medicine, 2016, 95(44): e5333.
doi: 10.1097/MD.0000000000005333 |
| [158] |
PIMENTEL-NUNES P, LIBÂNIO D, BASTIAANSEN B A J, et al. Endoscopic submucosal dissection for superficial gastrointestinal lesions: European Society of Gastrointestinal Endoscopy (ESGE) guideline-update 2022[J]. Endoscopy, 2022, 54(6): 591-622.
doi: 10.1055/a-1811-7025 |
| [159] | NAKAJIMA G, HAYASHI K, XI Y G, et al. Non-coding microRNAs hsa-let-7g and hsa-miR-181b are associated with chemoresponse to S-1 in colon cancer[J]. Cancer Genomics Proteomics, 2006, 3(5): 317-324. |
| [160] | 廖健南, 邱磊, 李日焕, 等. 肠镜活检方式对直肠癌术前病理组织学分级准确率的影响[J]. 中国内镜杂志, 2012, 18(1): 17-20. |
| LIAO J N, QIU L, LI R H, et al. Effect of colonoscopy biopsy way on preoperative histopathology grading accuracy assessment of rectal adenocarcinoma[J]. China J Endosc, 2012, 18(1): 17-20. | |
| [161] |
DEKKER E, TANIS P J, VLEUGELS J L A, et al. Colorectal cancer[J]. Lancet, 2019, 394(10207): 1467-1480.
doi: S0140-6736(19)32319-0 pmid: 31631858 |
| [162] |
MOORE J S, AULET T H. Colorectal Cancer Screening[J]. Surg Clin North Am, 2017, 97(3): 487-502.
doi: S0039-6109(17)30001-4 pmid: 28501242 |
| [163] |
XU R H, SHEN L, LI J, et al. Expert consensus on maintenance treatment for metastatic colorectal cancer in China[J]. Chin J Cancer, 2016, 35: 13.
doi: 10.1186/s40880-015-0067-x |
| [164] |
BILLER L H, SCHRAG D. Diagnosis and treatment of metastatic colorectal cancer: a review[J]. JAMA, 2021, 325(7): 669-685.
doi: 10.1001/jama.2021.0106 pmid: 33591350 |
| [165] |
PATEL S G, KARLITZ J J, YEN T, et al. The rising tide of early-onset colorectal cancer: a comprehensive review of epidemiology, clinical features, biology, risk factors, prevention, and early detection[J]. Lancet Gastroenterol Hepatol, 2022, 7(3): 262-274.
doi: 10.1016/S2468-1253(21)00426-X |
| [166] |
KUDO S E, ICHIMASA K, VILLARD B, et al. Artificial intelligence system to determine risk of T1 colorectal cancer metastasis to lymph node[J]. Gastroenterology, 2021, 160(4): 1075-1084.e2.
doi: 10.1053/j.gastro.2020.09.027 |
| [167] |
BACKES Y, MOSS A, REITSMA J B, et al. Narrow band imaging, magnifying chromoendoscopy, and gross morphological features for the optical diagnosis of T1 colorectal cancer and deep submucosal invasion: a systematic review and meta-analysis[J]. Am J Gastroenterol, 2017, 112(1): 54-64.
doi: 10.1038/ajg.2016.403 |
| [168] |
SAGAERT X, VANSTAPEL A, VERBEEK S. Tumor heterogeneity in colorectal cancer: what do we know so far?[J]. Pathobiology, 2018, 85(1/2): 72-84.
doi: 10.1159/000486721 |
| [169] |
SIMON K. Colorectal cancer development and advances in screening[J]. Clin Interv Aging, 2016, 11: 967-976.
doi: 10.2147/CIA.S109285 pmid: 27486317 |
| [170] |
LUGLI A, ZLOBEC I, BERGER M D, et al. Tumour budding in solid cancers[J]. Nat Rev Clin Oncol, 2021, 18(2): 101-115.
doi: 10.1038/s41571-020-0422-y |
| [171] |
BROUWER N P M, NAGTEGAAL I D. Tumor deposits improve staging in colon cancer: what are the next steps?[J]. Ann Oncol, 2021, 32(10): 1209-1211.
doi: 10.1016/j.annonc.2021.08.1751 pmid: 34416364 |
| [172] | MINSKY B D, RODEL C. Identifying the most predictive post-chemoradiation TRG system for rectal cancer[J]. J Natl Cancer Inst, 2014, 106(10): dju285. |
| [173] |
BOLAND C R, GOEL A. Microsatellite instability in colorectal cancer[J]. Gastroenterology, 2010, 138(6): 2073-2087.e3.
doi: 10.1053/j.gastro.2009.12.064 pmid: 20420947 |
| [174] | National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology, colon cancer, version 4. 2023[EB/OL]. [2023-11-25]. https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf. |
| [175] | National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology, rectal cancer, version 6. 2023[EB/OL]. [2023-11-25]. https://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf. |
| [176] |
KEKELIDZE M, D'ERRICO L, PANSINI M, et al. Colorectal cancer: current imaging methods and future perspectives for the diagnosis, staging and therapeutic response evaluation[J]. World J Gastroenterol, 2013, 19(46): 8502-8514.
doi: 10.3748/wjg.v19.i46.8502 |
| [177] |
BENSON A B, VENOOK A P, AL-HAWARY M M, et al. Rectal cancer, version 2.2018, NCCN clinical practice guidelines in oncology[J]. J Natl Compr Canc Netw, 2018, 16(7): 874-901.
doi: 10.6004/jnccn.2018.0061 |
| [178] |
GROUP M S. Diagnostic accuracy of preoperative magnetic resonance imaging in predicting curative resection of rectal cancer: prospective observational study[J]. BMJ, 2006, 333(7572): 779.
doi: 10.1136/bmj.38937.646400.55 |
| [179] |
HUNDT W, BRAUNSCHWEIG R, REISER M. Evaluation of spiral CT in staging of colon and rectum carcinoma[J]. Eur Radiol, 1999, 9(1): 78-84.
doi: 10.1007/s003300050632 pmid: 9933385 |
| [180] |
CASH B D, ROCKEY D C, BRILL J V. AGA standards for gastroenterologists for performing and interpreting diagnostic computed tomography colonography: 2011 update[J]. Gastroenterology, 2011, 141(6): 2240-2266.
doi: 10.1053/j.gastro.2011.09.043 pmid: 22098711 |
| [181] |
ZALIS M E, BARISH M A, et al. CT colonography reporting and data system: a consensus proposal[J]. Radiology, 2005, 236(1): 3-9.
doi: 10.1148/radiol.2361041926 pmid: 15987959 |
| [182] |
PICKHARDT P J, KIM D H, POOLER B D, et al. Assessment of volumetric growth rates of small colorectal polyps with CT colonography: a longitudinal study of natural history[J]. Lancet Oncol, 2013, 14(8): 711-720.
doi: 10.1016/S1470-2045(13)70216-X pmid: 23746988 |
| [183] |
PICKHARDT P J, POOLER B D, MBAH I, et al. Colorectal findings at repeat CT colonography screening after initial CT colonography screening negative for polyps larger than 5 mm[J]. Radiology, 2017, 282(1): 139-148.
doi: 10.1148/radiol.2016160582 pmid: 27552558 |
| [184] |
VAN DER PAARDT M P, STOKER J. Current status of magnetic resonance colonography for screening and diagnosis of colorectal cancer[J]. Radiol Clin North Am, 2018, 56(5): 737-749.
doi: 10.1016/j.rcl.2018.04.007 |
| [185] |
OFFERMANS T, VOGELAAR F J, AQUARIUS M, et al. Preoperative segmental localization of colorectal carcinoma: CT colonography vs optical colonoscopy[J]. Eur J Surg Oncol, 2017, 43(11): 2105-2111.
doi: 10.1016/j.ejso.2017.09.016 |
| [186] |
FURUKAWA H, IKUMA H, SEKI A, et al. Positron emission tomography scanning is not superior to whole body multidetector helical computed tomography in the preoperative staging of colorectal cancer[J]. Gut, 2006, 55(7): 1007-1011.
pmid: 16361308 |
| [187] |
BRUSH J, BOYD K, CHAPPELL F, et al. The value of FDG positron emission tomography/computerised tomography (PET/CT) in pre-operative staging of colorectal cancer: a systematic review and economic evaluation[J]. Health Technol Assess, 2011, 15(35): 1-192, Ⅲ-Ⅳ.
doi: 10.3310/hta15350 pmid: 21958472 |
| [188] |
LIANIDOU E S, MAVROUDIS D, SOTIROPOULOU G, et al. What’ s new on circulating tumor cells? A meeting report[J]. Breast Cancer Res, 2010, 12(4): 307.
doi: 10.1186/bcr2601 |
| [189] |
LIU M C, OXNARD G R, KLEIN E A, et al. Sensitive and specific multi-cancer detection and localization using methylation signatures in cell-free DNA[J]. Ann Oncol, 2020, 31(6): 745-759.
doi: 10.1016/j.annonc.2020.02.011 pmid: 33506766 |
| [190] |
CRISTIANO S, LEAL A, PHALLEN J, et al. Genome-wide cell-free DNA fragmentation in patients with cancer[J]. Nature, 2019, 570(7761): 385-389.
doi: 10.1038/s41586-019-1272-6 |
| [191] |
KAMBOJ A K, AGARWAL S, CHEDID V G, et al. Changes in national google trends and local healthcare utilization after high-impact gastroenterology publications[J]. Am J Gastroenterol, 2021, 116(12): 2465-2469.
doi: 10.14309/ajg.0000000000001516 pmid: 34534126 |
| [192] |
DEIBEL A, DENG L, CHENG C Y, et al. Evaluating key characteristics of ideal colorectal cancer screening modalities: the microsimulation approach[J]. Gastrointest Endosc, 2021, 94(2): 379-390.e7.
doi: 10.1016/j.gie.2021.02.013 pmid: 33600806 |
| [193] | ZENG C, STROUP E K, ZHANG Z, et al. Towards precision medicine: advances in 5-hydroxymethylcytosine cancer biomarker discovery in liquid biopsy[J]. Cancer Commun (Lond), 2019, 39(1): 12. |
| [194] |
CAI J B, CHEN L, ZHANG Z, et al. Genome-wide mapping of 5-hydroxymethylcytosines in circulating cell-free DNA as a non-invasive approach for early detection of hepatocellular carcinoma[J]. Gut, 2019, 68(12): 2195-2205.
doi: 10.1136/gutjnl-2019-318882 pmid: 31358576 |
| [195] |
GULER G D, NING Y H, KU C J, et al. Detection of early stage pancreatic cancer using 5-hydroxymethylcytosine signatures in circulating cell free DNA[J]. Nat Commun, 2020, 11: 5270.
doi: 10.1038/s41467-020-18965-w pmid: 33077732 |
| [196] | LIU T, CHANG W, YE W, et al. Detection of 5-hydroxymethylcytosine in circulating-free DNA for early diagnosis of colorectal cancer[J]. Ann Oncol, 2019, 30(Suppl 5). |
| [197] |
PEGTEL D M, GOULD S J. Exosomes[J]. Annu Rev Biochem, 2019, 88: 487-514.
doi: 10.1146/annurev-biochem-013118-111902 pmid: 31220978 |
| [198] |
BARTEL D P. MicroRNAs: genomics, biogenesis, mechanism, and function[J]. Cell, 2004, 116(2): 281-297.
doi: 10.1016/s0092-8674(04)00045-5 pmid: 14744438 |
| [199] |
RAUT J R, SCHÖTTKER B, HOLLECZEK B, et al. A microRNA panel compared to environmental and polygenic scores for colorectal cancer risk prediction[J]. Nat Commun, 2021, 12(1): 4811.
doi: 10.1038/s41467-021-25067-8 pmid: 34376648 |
| [200] | 窦利州, 张月明, 贺舜, 等. 早期结直肠癌内镜下治疗的远期疗效分析[J]. 中华肿瘤杂志, 2020, 42(9): 758-764. |
| DOU L Z, ZHANG Y M, HE S, et al. Long-term outcome after endoscopic resection for early colorectal carcinoma[J]. Chin J Oncol, 2020, 42(9): 758-764. | |
| [201] | 中华医学会消化内镜学分会, 中国抗癌协会肿瘤内镜学专业委员会. 中国早期结直肠癌筛查及内镜诊治指南(2014年,北京)[J]. 胃肠病学, 2015, 20(6): 345-365. |
| Digestive Endoscopy Branch of Chinese Medical Association, Cancer Endoscopy Professional Committee of China Anti-Cancer Association. Guidelines for Screening and Endoscopic Diagnosis and Treatment of Early Colorectal Cancer in China (2014, Beijing)[J]. Chin J Gastroenter, 2015, 20(6): 345-365. | |
| [202] | CARRARA A, GHEZZI G, REICH F, et al. Risk factors for nodal involvement in early-stage rectal cancer: a new scoring system based on the analysis of 326 cases[J]. Minerva Surg, 2022, 77(5): 448-454. |
| [203] | 张北平, 魏玮, 李爱民, 等. 结直肠腺瘤及早期结直肠癌中西医结合诊治专家共识(2021)[J]. 中医杂志, 2022, 63(10): 989-997. |
| ZHANG B P, WEI W, LI A M. Expert consensus on diagnosis and treatment of colorectal adenoma and early colorectal cancer with integrated traditional Chinese and western medicine (2021)[J]. J Tradit Chin Med, 2022, 63(10): 989-997. | |
| [204] | 中华医学会消化内镜学分会消化系早癌内镜诊断与治疗协作组, 中华医学会消化病学分会消化道肿瘤协作组, 中华医学会消化内镜学分会肠道学组, 等. 中国早期结直肠癌及癌前病变筛查与诊治共识意见(2014年11月·重庆)[J]. 中华内科杂志, 2015, 54(4): 375-389. |
| Collaborative Group on Endoscopic Diagnosis and Treatment of Early Gastrointestinal Cancer of Digestive Endoscopy Branch of Chinese Medical Association, Digestive Tumor Collaborative Group of the Digestive Disease Branch of Chinese Medical Association, Gastroenterology Group of Digestive Endoscopy Branch of Chinese Medical Association, et al. Consensus on screening, diagnosis and treatment of early colorectal cancer and precancerous lesions in China (Chongqing, November 2014)[J]. Chin J Intern Med, 2015, 54(4): 375-389. | |
| [205] |
WITJES C D M, PATEL A S, SHENOY A, et al. Oncological outcome after local treatment for early stage rectal cancer[J]. Surg Endosc, 2022, 36(1): 489-497.
doi: 10.1007/s00464-021-08308-1 |
| [206] |
METZ A J, MOSS A, MCLEOD D, et al. A blinded comparison of the safety and efficacy of hot biopsy forceps electrocauterization and conventional snare polypectomy for diminutive colonic polypectomy in a porcine model[J]. Gastrointest Endosc, 2013, 77(3): 484-490.
doi: 10.1016/j.gie.2012.09.014 pmid: 23199650 |
| [207] |
POHL H, SRIVASTAVA A, BENSEN S P, et al. Incomplete polyp resection during colonoscopy-results of the complete adenoma resection (CARE) study[J]. Gastroenterology, 2013, 144(1): 74-80.e1.
doi: 10.1053/j.gastro.2012.09.043 pmid: 23022496 |
| [208] |
SAKAMOTO T, MATSUDA T, OTAKE Y, et al. Predictive factors of local recurrence after endoscopic piecemeal mucosal resection[J]. J Gastroenterol, 2012, 47(6): 635-640.
doi: 10.1007/s00535-011-0524-5 pmid: 22223177 |
| [209] |
KIRIYAMA S, SAITO Y, YAMAMOTO S, et al. Comparison of endoscopic submucosal dissection with laparoscopic-assisted colorectal surgery for early-stage colorectal cancer: a retrospective analysis[J]. Endoscopy, 2012, 44(11): 1024-1030.
doi: 10.1055/s-0032-1310259 pmid: 23012216 |
| [210] |
NAKAJIMA T, SAITO Y, TANAKA S, et al. Current status of endoscopic resection strategy for large, early colorectal neoplasia in Japan[J]. Surg Endosc, 2013, 27(9): 3262-3270.
doi: 10.1007/s00464-013-2903-x pmid: 23508817 |
| [211] |
ZENG Q S, ZOU M, NIE J, et al. Efficacy and safety of endoscopic submucosal dissection for rectal tumors extending versus not to the dentate line: a systematic review and meta-analysis[J]. J Clin Gastroenterol, 2022, 56(6): 518-528.
doi: 10.1097/MCG.0000000000001692 |
| [212] |
BELDERBOS T D, LEENDERS M, MOONS L M, et al. Local recurrence after endoscopic mucosal resection of nonpedunculated colorectal lesions: systematic review and meta-analysis[J]. Endoscopy, 2014, 46(5): 388-402.
doi: 10.1055/s-0034-1364970 pmid: 24671869 |
| [213] | National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology, rectal cancer, version 1.2022[EB/OL]. (2022-02-25)[2023-11-25]. https://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf. |
| [214] |
TAN S, XU C, MA H, et al. Local resection versus radical resection for early-stage rectal cancer: a systematic review and meta-analysis[J]. Int J Colorectal Dis, 2022, 37(7): 1467-1483.
doi: 10.1007/s00384-022-04186-8 |
| [215] |
JOSEPH HOEPFL M D, et al. Limitations of early rectal cancer nodal staging may explain failure after local excision[J]. Dis Colon Rectum, 2007, 50(10): 1520-1525.
pmid: 17674104 |
| [216] |
NASH G M, WEISER M R, GUILLEM J G, et al. Long-term survival after transanal excision of T1 rectal cancer[J]. Dis Colon Rectum, 2009, 52(4): 577-582.
doi: 10.1007/DCR.0b013e3181a0adbd |
| [217] |
YOU Y N, BAXTER N N, STEWART A, et al. Is the increasing rate of local excision for stage Ⅰ rectal cancer in the United States justified? A nationwide cohort study from the National Cancer Database[J]. Ann Surg, 2007, 245(5): 726-733.
doi: 10.1097/01.sla.0000252590.95116.4f |
| [218] |
RICCARDO NASCIMBENI M D, SANTHAT NIVATVONGS M D, et al. Risk of lymph node metastasis in T1 carcinoma of the colon and rectum[J]. Dis Colon Rectum, 2002, 45(2): 200-206.
doi: 10.1007/s10350-004-6147-7 |
| [219] | 林国乐, 邱辉忠, 周皎琳, 等. 经肛门内镜微创手术的适应证与并发症[J]. 中华结直肠疾病电子杂志, 2015, 4(5): 63-67. |
| LIN G L, QIU H Z, ZHOU J L, et al. The indications and complications of transanal endoscopic microsurgery[J]. Chin J Colorectal Dis Electron Ed, 2015, 4(5): 63-67. | |
| [220] |
CLANCY C, BURKE J P, ALBERT M R, et al. Transanal endoscopic microsurgery versus standard transanal excision for the removal of rectal neoplasms: a systematic review and meta-analysis[J]. Dis Colon Rectum, 2015, 58(2): 254-261.
doi: 10.1097/DCR.0000000000000309 pmid: 25585086 |
| [221] |
CHEN Y, GUO R, XIE J, et al. Laparoscopy combined with transanal endoscopic microsurgery for rectal cancer: a prospective, single-blinded, randomized clinical trial[J]. Surg Laparosc Endosc Percutan Tech, 2015, 25(5): 399-402.
doi: 10.1097/SLE.0000000000000186 pmid: 26429049 |
| [222] |
KIDANE B, CHADI S A, KANTERS S, et al. Local resection compared with radical resection in the treatment of T1N0M0 rectal adenocarcinoma: a systematic review and meta-analysis[J]. Dis Colon Rectum, 2015, 58(1): 122-140.
doi: 10.1097/DCR.0000000000000293 |
| [223] |
SAJID M S, FARAG S, LEUNG P, et al. Systematic review and meta-analysis of published trials comparing the effectiveness of transanal endoscopic microsurgery and radical resection in the management of early rectal cancer[J]. Colorectal Dis, 2014, 16(1): 2-14.
doi: 10.1111/codi.12474 pmid: 24330432 |
| [224] |
LU J Y, LIN G L, QIU H Z, et al. Comparison of transanal endoscopic microsurgery and total mesorectal excision in the treatment of T1 rectal cancer: a meta-analysis[J]. PLoS One, 2015, 10(10): e0141427.
doi: 10.1371/journal.pone.0141427 |
| [225] | 中国NOSES联盟, 中国医师协会结直肠肿瘤专业委员会NOSES专委会. 结直肠肿瘤经自然腔道取标本手术专家共识(2017)[J]. 中华结直肠疾病电子杂志, 2017, 6(4): 266-272. |
| China NOSES Alliance, Professional Committee of Natural Orifice Specimen Extraction Surgery, Colorectal Cancer Committee of Chinese Medical Doctor Association. Expert consensus of natural orifice specimen extraction surgery in colorectal neoplasm (2017 edition)[J]. Chin J Colorectal Dis Electron Ed, 2017, 6(4): 266-272. | |
| [226] | 中国医师协会外科医师分会经肛门全直肠系膜切除术专业委员会, 中国医师协会外科医师分会结直肠外科医师委员会, 中国经肛腔镜外科学院, 等. 中国经肛腔镜手术专家共识(2019版)[J]. 中华胃肠外科杂志, 2019(6): 501-506. |
| Chinese Society of Transanal Total Mesoretal Excision, Chinese Society of Colon and Rectal Surgeons, Chinese Transanal Endoscopic Surgery College. Chinese consensus on transanal endoscopic surgery (2019 version)[J]. Chin J Gastrointest Surg, 2019(6): 501-506. | |
| [227] | 罗双灵, 康亮. taTME的学习曲线和规范化培训[J]. 结直肠肛门外科, 2022, 28(1): 10-13. |
| LUO S L, KANG L. The learning curve of transanal total mesorectal excision (taTME) and its standardized training[J]. J Colorectal Anal Surg, 2022, 28(1): 10-13. | |
| [228] | 张卫. 极低位直肠癌经括约肌间切除保肛手术的再认识[J]. 中华胃肠外科杂志, 2022, 25(6): 487-492. |
| ZHANG W. Reassessment of intersphincteric resection in the sphincter-preserving operation for ultra-low rectal cancer[J]. Chin J Gastrointest Surg, 2022, 25(6): 487-492. | |
| [229] |
SUN G, LOU Z, ZHANG H, et al. Retrospective study of the functional and oncological outcomes of conformal sphincter preservation operation in the treatment of very low rectal cancer[J]. Tech Coloproctol, 2020, 24(10): 1025-1034.
doi: 10.1007/s10151-020-02229-2 pmid: 32361871 |
| [230] | 孙戈, 臧怡雯, 丁海波, 等. 适形切除保肛术与经括约肌间切除术治疗低位直肠癌的临床疗效[J]. 中华消化外科杂志, 2021, 20(3): 292-300. |
| SUN G, ZANG Y W, DING H B, et al. Clinical efficacy of conformal sphincter preservation operation versus intersphincteric resection in the treatment of low rectal cancer[J]. Chin J Dig Surg, 2021, 20(3): 292-300. | |
| [231] | 朱晓明, 楼征, 白辰光, 等. 低位直肠癌拖出式适形切除术肠壁侧切缘安全距离的初步探讨[J]. 中华胃肠外科杂志, 2016, 19(9): 1025-1029. |
| ZHU X M, LOU Z, BAI C G, et al. Preliminary investigation of intramural lateral spread distance in pull-through conformal resection of low rectal cancer[J]. Chin J Gastrointest Surg, 2016, 19(9): 1025-1029. | |
| [232] |
HALLEMEIER C L. Who is a candidate for nonoperative management of early-stage rectal cancer?[J]. Clin Adv Hematol Oncol, 2022, 20(5): 281-283.
pmid: 35579585 |
| [233] | MATHEW R. Radical surgery versus organ preservation for early-stage rectal cancer[J]. Lancet Gastroenterol Hepatol, 2021, 6(4): 263. |
| [234] | 周乐其, 于冠宇, 沈钰新, 等. 程序性死亡蛋白-1抑制剂联合新辅助放化疗治疗微卫星稳定极低位直肠癌的安全性及其疗效[J]. 中华胃肠外科杂志, 2022, 25(3): 250-256. |
| ZHOU L Q, YU G Y, SHEN Y X, et al. Safety and efficacy of programmed death protein-1 inhibitor combined with neoadjuvant radiotherapy and chemotherapy in the treatment of microsatellite stabilized extremely low rectal cancer[J]. Chin J Gastrointest Surg, 2022, 25(3): 250-256. | |
| [235] |
ANDRE T, SHIU K K, KIM T W, et al. Pembrolizumab in microsatellite-instability-high advanced colorectal cancer[J]. N Engl J Med, 2020, 383(23): 2207-2218.
doi: 10.1056/NEJMoa2017699 |
| [236] |
KALBASI A, JUNE C H, HAAS N, et al. Radiation and immunotherapy: a synergistic combination[J]. J Clin Invest, 2013, 123(7): 2756-2763.
doi: 10.1172/JCI69219 pmid: 23863633 |
| [237] |
CHALABI M, FANCHI L F, DIJKSTRA K K, et al. Neoadjuvant immunotherapy leads to pathological responses in MMR-proficient and MMR-deficient early-stage colon cancers[J]. Nat Med, 2020, 26(4): 566-576.
doi: 10.1038/s41591-020-0805-8 pmid: 32251400 |
| [238] | 张卫, 颜宏利, 高显华. 早发性结直肠癌[M]. 上海: 上海科学技术出版社, 2022. |
| ZHANG W, YAN H L, GAO X H. Early onset colorectal cancer[M]. Shanghai: Shanghai Scientific and Technical Publishers, 2022. | |
| [239] | 中华医学会消化病学分会炎症性肠病学组. 炎症性肠病诊断与治疗的共识意见(2018年·北京)[J]. 中华炎性肠病杂志, 2018, 2(3): 173-190. |
| Inflammatory Bowel Disease Group, Chinese Society of Gastroenterology, Chinese Medical Association. Chinese consensus on diagnosis and treatment in inflammatory bowel disease (2018, Beijing)[J]. Chin J Inflamm Bowel Dis, 2018, 2(3): 173-190. | |
| [240] |
GONG W, LV N, WANG B, et al. Risk of ulcerative colitis-associated colorectal cancer in China: a multi-center retrospective study[J]. Dig Dis Sci, 2012, 57(2): 503-507.
doi: 10.1007/s10620-011-1890-9 |
| [241] | ZENG Q S, ZHAO Z J, NIE J, et al. Efficacy and safety of endoscopic submucosal dissection for dysplasia in ulcerative colitis patients: a systematic review and meta-analysis[J]. Gastroenterol Res Pract, 2022, 2022: 9556161. |
| [1] | HUANG Haozhe, CHEN Hong, ZHENG Dezhong, CHEN Chao, WANG Ying, XU Lichao, WANG Yaohui, HE Xinhong, YANG Yuanyuan, LI Wentao. A CT-based radiomics nomogram for predicting local tumor progression of colorectal cancer lung metastases treated with radiofrequency ablation [J]. China Oncology, 2024, 34(9): 857-872. |
| [2] | WU Wen, ZHANG Ruoxin, WENG Junyong, MA Yanlei, CAI Guoxiang, LI Xinxiang, YANG Yongzhi. Exploring the prognostic value of positive lymph node ratio in stage Ⅲ colorectal cancer patients and establishing a predictive model [J]. China Oncology, 2024, 34(9): 873-880. |
| [3] | GE Zuyin, SONG Kun, LIN Yunxiao, ZHONG Yeling, HAO Jingduo. The feasibility study of FCGBP and BIGH3 in circulating tumor cells as potential markers for colorectal cancer [J]. China Oncology, 2024, 34(8): 745-752. |
| [4] | WENG Junyong, YE Zilan, ZHANG Ruoxin, LIU Qi, LI Xinxiang. Exploring the guiding role of the number of adverse pathological features in risk stratification for recurrence of stage Ⅰ-Ⅲ colorectal cancer: a retrospective cohort study of 9 875 cases [J]. China Oncology, 2024, 34(6): 527-536. |
| [5] | Urologic Chinese Oncology Group. Expert consensus on early diagnosis and treatment of bladder cancer (2024 edition) [J]. China Oncology, 2024, 34(6): 607-618. |
| [6] | ZHANG Ruoxin, YE Zilan, WENG Junyong, LI Xinxiang. Correlation study between advanced age and inferior prognosis in stage Ⅱ colorectal cancer patients [J]. China Oncology, 2024, 34(5): 485-492. |
| [7] | LU Yue, LU Renquan, ZHANG Jie, ZHENG Hui. Application value of combined coagulation function indicators in monitoring hypercoagulable state of patients with colorectal cancer after chemotherapy [J]. China Oncology, 2024, 34(3): 278-285. |
| [8] | LI Jun, LU Tingwei, FANG Xuqian. Impact of MSI-H/dMMR on clinicopathological characteristics and prognosis of patients with BRAF V600E-mutated resectable colorectal cancer [J]. China Oncology, 2024, 34(11): 1061-1066. |
| [9] | WU Zhibai, XU Guiqin, ZHANG Li, YANG Zhaojuan, LIU Yun, JIAO Kun, CHEN Zehong, XU Chen, ZUO You, ZHENG Ningqian, YE Zhiqian, LIU Yongzhong. Mechanism study of KCMF1 promoting proliferation and NF-κB signaling transduction in colorectal cancer cells [J]. China Oncology, 2024, 34(11): 987-997. |
| [10] | ZHANG Shaohua, LI Zhening, WANG Wei, WEI Yifan, HONG Yonggang, HAO Liqiang. Research progress in the related treatment of KRAS mutant colorectal cancer [J]. China Oncology, 2024, 34(10): 979-986. |
| [11] | WU Han, XU Lei, WANG Miaomiao, ZHANG Ruizhe, XU Xiaoyang, GUO Ningjie, WU Shuhua. Correlation of LC3 and the recruitment of dendritic cell and the formation of TLS in colorectal cancer and its clinical significance [J]. China Oncology, 2023, 33(9): 818-828. |
| [12] | ZHOU Cong, HE Lina, CHENG Xiaojiao, HUANG Tinglei, TU Shuiping. Effect of RSPO3 on inhibiting the growth of colorectal cancer transplanted tumors and increasing NK cell infiltration in vivo [J]. China Oncology, 2023, 33(7): 664-672. |
| [13] | YE Junling, ZHENG Xiaoying, GUO Xinjian, CHEN Ruihui, YANG Liu, GOU Xiaodan, JIANG Hanmei. A study on mechanism of lncRNA-mediated SNHG5/miR-26a-5p/MTDH signal axis promoting metastasis of colorectal cancer [J]. China Oncology, 2023, 33(7): 673-685. |
| [14] | CAI Jialuo, ZHU Ruiqiu, LI Sen, CAO Yijun, HUANG Fang. Mechanism of inflammatory cancer-associated fibroblast-mediated drug resistance in colorectal cancer cells [J]. China Oncology, 2023, 33(12): 1065-1072. |
| [15] | The Society of Breast Cancer, China Anti-Cancer Association. Screening and early diagnosis of breast cancer in China: a practice guideline [J]. China Oncology, 2022, 32(4): 363-372. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
沪ICP备12009617
Powered by Beijing Magtech Co. Ltd
