Welcome to China Oncology,
China Oncology ›› 2023, Vol. 33 ›› Issue (10): 936-944.doi: 10.19401/j.cnki.1007-3639.2023.10.006
• Review • Previous Articles Next Articles
Received:2023-05-16
Revised:2023-10-01
Online:2023-10-30
Published:2023-10-31
Contact:
ZHU Guannan.
Share article
CLC Number:
ZHAO Bolun, ZHU Guannan. Advances in the treatment of advanced melanoma with NRAS gene mutation[J]. China Oncology, 2023, 33(10): 936-944.
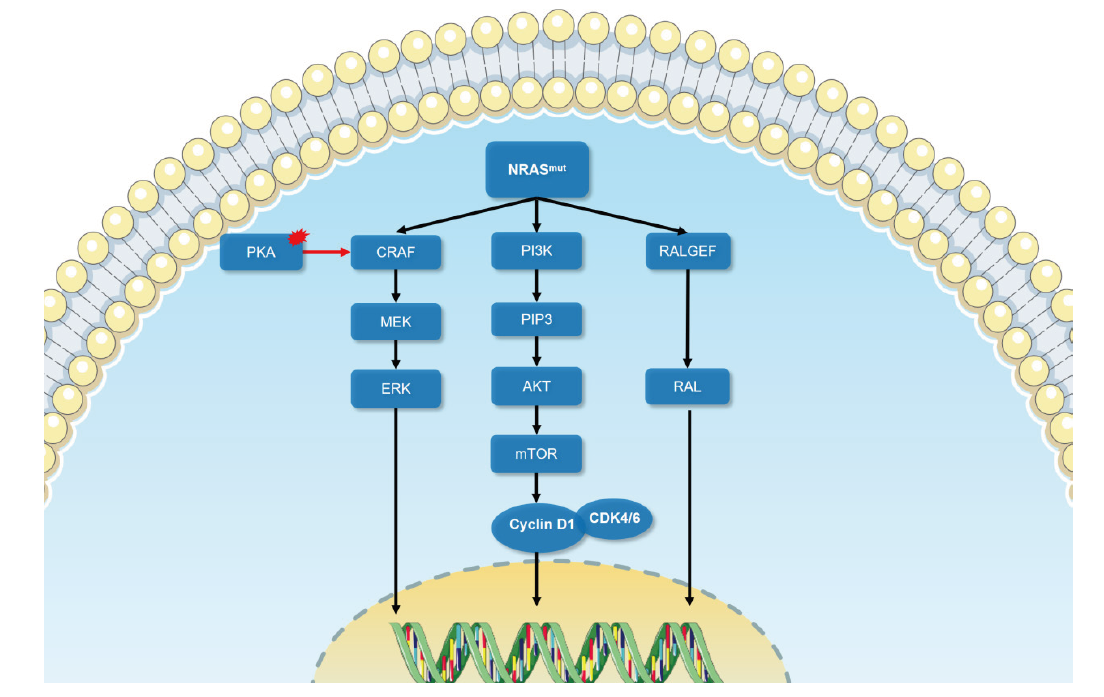
Fig. 1
The schematic diagram of signal transduction of NRAS mutation Oncogenic signal transduction following NRAS mutation causes melanoma development mainly through MAPK signaling, PI3K-AKT signaling pathway and RAS-RAL signaling pathway. Black arrow: Conducted; Red arrow: Enhanced; Red area: Abnormal activation."
Tab. 1
Treatment of NRAS mutant melanoma in clinical trials"
| Identifier | Target | Study design | NRAS-mutant mlanoma patients | Efficacy | Time frame |
|---|---|---|---|---|---|
| NCT03013101, NCT02821000, NCT02738489, CTR20160872[ | PD-1 antiboby | Clinical data from four clinical trials in patients with advanced melanoma treated with anti-PD-1 monoclonal antibodies between 2016 and 2019 were analyzed. The efficacy of immunotherapy in patients with cutaneous and non-cutaneous NRAS mutant melanoma was analyzed separately. | A total of 206 patients were assessed, including 12 patients with NRAS-mutated cutaneous melanoma and 21 patients with NRAS-mutated non-cutaneous melanoma | In cutaneous melanoma, patients with NRAS mutations had a lower overall response rate (ORR) than patients without NRAS mutations (9.5% vs 23.9%). In non-cutaneous melanoma, response rates were 0% and 13.7%, median progression-free survival (mPFS) was 3.6 months and 4.3 months (P=0.015), and median survival time (mOS) was 10.8 months and 15.3 months (P=0.025) in NRAS mutant and wild-type patients, respectively | 2016-2019 |
| NCT01763164[ | MEKi binimetinib | Phase Ⅲ randomized, multicenter, open-label clinical trial. Patients with advanced, unresectable stage ⅢC-Ⅳ NRAS mutated melanoma who were previously untreated or progressed following prior immunotherapy were randomized (2:1) to receive binimetinib 45 mg orally twice daily or dacarbazine 1 000 mg/m2 intravenously every 3 weeks. | A total of 402 patients with NRAS-mutated melanoma were enrolled, 269 treated with binimetinib and 233 treated with dacarbazine (1:2) | In the binimetinib arm mPFS was 2.8 months and 1.5 months in the dacarbazine arm | 2013-2015 |
| NCT01693068[ | MEKi pmasertib | Phase Ⅱ multicenter, open-label clinical trial. Patients with unresectable stage Ⅲc/ⅣM1 NRAS-mutated cutaneous melanoma were randomized 2:1 to receive pmasertib (60 mg orally twice daily) or DTIC (1 000 mg/m2 intravenously). Primary endpoint: investigator-assessed PFS; secondary endpoints: OS, ORR, quality of life (QoL), and safety. | 191 patients with NRAS mutated cutaneous melanoma, 191 treated (pimasertib n=130, DTIC n=61) | PFS and 6-month PFS rates were significantly improved in the pimasertib arm compared with the DTIC arm: 13 weeks versus 7 weeks, 17% vs 9%. Investigator-assessed ORR was 27% in the pimasertib arm and 14% in the DTIC arm. However, there was no difference in OS between patients treated with pimasertib and DTIC (mOS 9 and 11 months, respectively; HR=0.89, 95% CI: 0.61-1.30) | 2012-2014 |
| NCT00060125[ | Farnesyltransferase inhibitor (FTI) | Three-stage trial design, up to 40 patients, stopped early if first 14 patients did not respond, or first 28 patients had less than 2 responders | 14 patients with NRAS mutated melanoma | 2 patients presented with grade 3 toxicity and all patients had no clinical response and the trial was prematurely discontinued | 2003-2006 |
| Identifier | Target | Study design | NRAS-mutant mlanoma patients | Efficacy | Time frame |
| NCT03118817[ | RAFi belvarafenib | Single-arm, open-label, multicenter, phase Ⅰ extension study | 9 NRAS mutated melanoma patients | ORR 11%, mPFS 25 weeks | 2017-2020 |
| NCT03973151[ | MEKi HL-085 | Phase Ⅰ/Ⅱ, single-arm, dose-escalation and cohort expansion study | 42 patients with NRAS mutated melanoma | HL-085 was published in 2023 confirming an ORR of 34.7% | 2019-2023 |
| NCT02974725[ | BRAF/CRAF protein kinases inhibitor Naporafenib(LXH254) + MEKi trametinib | Phase Ⅰb escalation/expansion study | 30 patients with NRAS mutated melanoma | The ORR was 46.7%, the median DOR was 3.75 and the overall median PFS was 5.52 months in patients treated with naporafenib 200 mg twice a day plus trametinib 1 mg once daily. | 2017-2023 |
| NCT01449058[ | PI3Kαinhibitor BYL719 +MEKi binimetinib | Phase Ⅰb open-label, multicenter, dose escalation and expansion study | 5 NRAS mutated melanoma patients | ORR 20% | 2011-2017 |
| NCT01941927[ | MEKi trametinib +AKT inhibitor GSK2141795 | Phase Ⅱ non-randomized, multicenter, open-label clinical trial | Efficacy and safety of MEK inhibitors combined with AKT inhibitors in 10 patients with NRAS-mutated melanoma and 10 patients with BRAFWT/NRASWT melanoma | The mPFS and mOS of the 10 NRAS-mutated melanoma patients were only 2.3 and 4.0 months. Median PFS and OS for the wild-type cohort were 2.8 months and 3.5 months, respectively. No objective responses were identified in either cohort. The combination of Trametinib and GSK2141795 has no significant clinical activity in NRAS mutants or BRAFWT/NRASWT melanomas | 2013-2020 |
| NCT03932253 | MEKi FCN-159 | Phase Ⅰa/Ⅰb, open-label, dose escalation and dose expansion study | 33 patients with NRAS mutated melanoma were enrolled | The ORR and clinical benefit rates were 19.0% and 52.4%, respectively. Median duration of response and progression-free survival were 4.8 months and 3.8 months (1.8-5.6 months), respectively. | 2019-2023 |
| NCT03284502 | MEKi cobimetinib + RAFi HM95573 | Phase Ⅰb, open-label, multicenter dose escalation study | 9 NRAS mutated melanoma patients | Available data published ORR of 40% | 2017-2023 |
| NCT03979651 | MEKi trametinib+autophagy inhibitor hydroxychloroquine | Phase Ⅰb/Ⅱ non-randomized, open-label clinical trial | 29 NRAS mutated melanoma patients | Results to be further published | 2019-2022 |
| NCT04109456 | MEKi cobimetinib | Phase Ⅰb open-label clinical study | Estimated enrollment is 120 patients with NRAS mutated melanoma | Results to be further published | 2019-2023 |
| [1] |
TSAI F D, LOPES M S, ZHOU M, et al. KRAS4A splice variant is widely expressed in cancer and uses a hybrid membrane-targeting motif[J]. Proc Natl Acad Sci U S A, 2015, 112(3): 779-784.
doi: 10.1073/pnas.1412811112 |
| [2] |
MACARA I G. The ras superfamily of molecular switches[J]. Cell Signal, 1991, 3(3): 179-187.
pmid: 1892732 |
| [3] |
JAKOB J A, BASSETT R L Jr, NG C S, et al. NRAS mutation status is an independent prognostic factor in metastatic melanoma[J]. Cancer, 2012, 118(16): 4014-4023.
doi: 10.1002/cncr.26724 |
| [4] | 曾颖, 康晓静, 张祥月, 等. 肢端型黑素瘤NRAS基因突变检测及预后分析[J]. 中华皮肤科杂志, 2016, 49(7): 474-477. |
| [5] |
LALLY S E, MILMAN T, ORLOFF M, et al. Mutational landscape and outcomes of conjunctival melanoma in 101 patients[J]. Ophthalmology, 2022, 129(6): 679-693.
doi: 10.1016/j.ophtha.2022.01.016 |
| [6] |
LISZKAY G, MÁTRAI Z, CZIRBESZ K, et al. Predictive and prognostic value of BRAF and NRAS mutation of 159 sentinel lymph node cases in melanoma-a retrospective single-institute study[J]. Cancers (Basel), 2021, 13(13): 3302.
doi: 10.3390/cancers13133302 |
| [7] |
ZABLOCKA T, KREISMANE M, PJANOVA D, et al. Effects of BRAF V600E and NRAS mutational status on the progression-free survival and clinicopathological characteristics of patients with melanoma[J]. Oncol Lett, 2023, 25(1): 27.
doi: 10.3892/ol |
| [8] |
DUMAZ N, HAYWARD R, MARTIN J, et al. In melanoma, RAS mutations are accompanied by switching signaling from BRAF to CRAF and disrupted cyclic AMP signaling[J]. Cancer Res, 2006, 66(19): 9483-9491.
doi: 10.1158/0008-5472.CAN-05-4227 |
| [9] | PHADKE M S, SMALLEY K S M. Targeting NRAS mutations in advanced melanoma[J]. J Clin Oncol, 2023, 41(14): 2661-2664. |
| [10] |
SMALLEY K S, HAASS N K, BRAFFORD P A, et al. Multiple signaling pathways must be targeted to overcome drug resistance in cell lines derived from melanoma metastases[J]. Mol Cancer Ther, 2006, 5(5): 1136-1144.
pmid: 16731745 |
| [11] |
SHARMA A, TRIVEDI N R, ZIMMERMAN M A, et al. Mutant V599EB-Raf regulates growth and vascular development of malignant melanoma tumors[J]. Cancer Res, 2005, 65(6): 2412-2421.
doi: 10.1158/0008-5472.CAN-04-2423 |
| [12] | SATYAMOORTHY K, LI G, GERRERO M R, et al. Constitutive mitogen-activated protein kinase activation in melanoma is mediated by both BRAF mutations and autocrine growth factor stimulation[J]. Cancer Res, 2003, 63(4): 756-759. |
| [13] |
ZHUANG L, LEE C S, SCOLYER R A, et al. Activation of the extracellular signal regulated kinase (ERK) pathway in human melanoma[J]. J Clin Pathol, 2005, 58(11): 1163-1169.
doi: 10.1136/jcp.2005.025957 pmid: 16254105 |
| [14] |
OBA J, NAKAHARA T, ABE T, et al. Expression of c-Kit, p-ERK and cyclin D1 in malignant melanoma: an immunohistochemical study and analysis of prognostic value[J]. J Dermatol Sci, 2011, 62(2): 116-123.
doi: 10.1016/j.jdermsci.2011.02.011 pmid: 21454057 |
| [15] |
CANCER GENOME ATLAS NETWORK. Genomic classification of cutaneous melanoma[J]. Cell, 2015, 161(7): 1681-1696.
doi: 10.1016/j.cell.2015.05.044 pmid: 26091043 |
| [16] |
ROBERTSON G P. Functional and therapeutic significance of Akt deregulation in malignant melanoma[J]. Cancer Metastasis Rev, 2005, 24(2): 273-285.
doi: 10.1007/s10555-005-1577-9 |
| [17] |
XIE X Q, WHITE E P, MEHNERT J M. Coordinate autophagy and mTOR pathway inhibition enhances cell death in melanoma[J]. PLoS One, 2013, 8(1): e55096.
doi: 10.1371/journal.pone.0055096 |
| [18] |
ZIPFEL P A, BRADY D C, KASHATUS D F, et al. Ral activation promotes melanomagenesis[J]. Oncogene, 2010, 29(34): 4859-4864.
doi: 10.1038/onc.2010.224 pmid: 20562921 |
| [19] |
MISHRA P J, HA L, RIEKER J, et al. Dissection of RAS downstream pathways in melanomagenesis: a role for Ral in transformation[J]. Oncogene, 2010, 29(16): 2449-2456.
doi: 10.1038/onc.2009.521 |
| [20] |
FALSETTI S C, WANG D A, PENG H R, et al. Geranylgeranyltransferase Ⅰ inhibitors target RalB to inhibit anchorage-dependent growth and induce apoptosis and RalA to inhibit anchorage-independent growth[J]. Mol Cell Biol, 2007, 27(22): 8003-8014.
doi: 10.1128/MCB.00057-07 |
| [21] |
SINGHAL S S, AWASTHI Y C, AWASTHI S. Regression of melanoma in a murine model by RLIP76 depletion[J]. Cancer Res, 2006, 66(4): 2354-2360.
pmid: 16489041 |
| [22] | MARTÍN M T, ALCALDE M, PLOU F J, et al. Covalent immobilization of cyclodextrin glucosyltransferase (CGTase) in activated silica and Sepharose[J]. Indian J Biochem Biophys, 2002, 39(4): 229-234. |
| [23] |
SWETTER S M, THOMPSON J A, ALBERTINI M R, et al. NCCN guidelines® insights: melanoma: cutaneous, version 2.2021[J]. J Natl Compr Canc Netw, 2021, 19(4): 364-376.
doi: 10.6004/jnccn.2021.0018 |
| [24] |
MICHIELIN O, VAN AKKOOI A C J, ASCIERTO P A, et al. Cutaneous melanoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up[J]. Ann Oncol, 2019, 30(12): 1884-1901.
doi: S0923-7534(20)32563-1 pmid: 31987293 |
| [25] | JAEGER Z J, RAVAL N S, MAVERAKIS N K A, et al. Objective response to immune checkpoint inhibitor therapy in NRAS-mutant melanoma: a systematic review and meta-analysis[J]. Front Med (Lausanne), 2023, 10: 1090737. |
| [26] |
ZHOU L, WANG X, CHI Z H, et al. Association of NRAS mutation with clinical outcomes of anti-PD-1 monotherapy in advanced melanoma: a pooled analysis of four Asian clinical trials[J]. Front Immunol, 2021, 12: 691032.
doi: 10.3389/fimmu.2021.691032 |
| [27] |
DUMMER R, SCHADENDORF D, ASCIERTO P A, et al. Binimetinib versus dacarbazine in patients with advanced NRAS-mutant melanoma (NEMO): a multicentre, open-label, randomised, phase 3 trial[J]. Lancet Oncol, 2017, 18(4): 435-445.
doi: 10.1016/S1470-2045(17)30180-8 |
| [28] |
LEBBÉ C, DUTRIAUX C, LESIMPLE T, et al. Pimasertib versus dacarbazine in patients with unresectable NRAS-mutated cutaneous melanoma: phase Ⅱ, randomized, controlled trial with crossover[J]. Cancers (Basel), 2020, 12(7): 1727.
doi: 10.3390/cancers12071727 |
| [29] |
RYAN M B, CORCORAN R B. Therapeutic strategies to target RAS-mutant cancers[J]. Nat Rev Clin Oncol, 2018, 15(11): 709-720.
doi: 10.1038/s41571-018-0105-0 pmid: 30275515 |
| [30] |
GAJEWSKI T F, SALAMA A K S, NIEDZWIECKI D, et al. Phase Ⅱ study of the farnesyltransferase inhibitor R115777 in advanced melanoma (CALGB 500104)[J]. J Transl Med, 2012, 10: 246.
doi: 10.1186/1479-5876-10-246 |
| [31] |
FEDORENKO I V, GIBNEY G T, SMALLEY K M. NRAS mutant melanoma: biological behavior and future strategies for therapeutic management[J]. Oncogene, 2013, 32(25): 3009-3018.
doi: 10.1038/onc.2012.453 |
| [32] |
TATEISHI K, TSUBAKI M, TAKEDA T, et al. FTI-277 and GGTI-289 induce apoptosis via inhibition of the Ras/ERK and Ras/mTOR pathway in head and neck carcinoma HEp-2 and HSC-3 cells[J]. J BUON, 2021, 26(2): 606-612.
pmid: 34077012 |
| [33] |
SKOULIDIS F, LI B T, DY G K, et al. Sotorasib for lung cancers with KRAS p.G12C mutation[J]. N Engl J Med, 2021, 384(25): 2371-2381.
doi: 10.1056/NEJMoa2103695 |
| [34] |
HONG D S, FAKIH M G, STRICKLER J H, et al. KRASG12C inhibition with sotorasib in advanced solid tumors[J]. N Engl J Med, 2020, 383(13): 1207-1217.
doi: 10.1056/NEJMoa1917239 |
| [35] |
JAISWAL B S, JANAKIRAMAN V, KLJAVIN N M, et al. Combined targeting of BRAF and CRAF or BRAF and PI3K effector pathways is required for efficacy in NRAS mutant tumors[J]. PLoS One, 2009, 4(5): e5717.
doi: 10.1371/journal.pone.0005717 |
| [36] |
YEN I, SHANAHAN F, LEE J, et al. ARAF mutations confer resistance to the RAF inhibitor belvarafenib in melanoma[J]. Nature, 2021, 594(7863): 418-423.
doi: 10.1038/s41586-021-03515-1 |
| [37] |
WANG X, LUO Z G, CHEN J, et al. First-in-human phase I dose-escalation and dose-expansion trial of the selective MEK inhibitor HL-085 in patients with advanced melanoma harboring NRAS mutations[J]. BMC Med, 2023, 21(1): 2.
doi: 10.1186/s12916-022-02669-7 |
| [38] |
ADAM C, FUSI L, WEISS N, et al. Efficient suppression of NRAS-driven melanoma by Co-inhibition of ERK1/2 and ERK5 MAPK pathways[J]. J Invest Dermatol, 2020, 140(12): 2455-2465.e10.
doi: 10.1016/j.jid.2020.03.972 |
| [39] |
CARLINO M S, TODD J R, GOWRISHANKAR K, et al. Differential activity of MEK and ERK inhibitors in BRAF inhibitor resistant melanoma[J]. Mol Oncol, 2014, 8(3): 544-554.
doi: 10.1016/j.molonc.2014.01.003 |
| [40] |
MENDZELEVSKI B, FERBER G, JANKU F, et al. Effect of ulixertinib, a novel ERK1/2 inhibitor, on the QT/QTc interval in patients with advanced solid tumor malignancies[J]. Cancer Chemother Pharmacol, 2018, 81(6): 1129-1141.
doi: 10.1007/s00280-018-3564-1 |
| [41] |
ATEFI M, TITZ B, AVRAMIS E, et al. Combination of pan-RAF and MEK inhibitors in NRAS mutant melanoma[J]. Mol Cancer, 2015, 14(1): 27.
doi: 10.1186/s12943-015-0293-5 |
| [42] | DE BRAUD F, DOOMS C, HEIST R S, et al. Initial evidence for the efficacy of naporafenib in combination with trametinib in NRAS-mutant melanoma: results from the expansion arm of a phase ib, open-label study[J]. J Clin Oncol, 2023, 41(14): 2651-2660. |
| [43] |
POSCH C, MOSLEHI H, FEENEY L, et al. Combined targeting of MEK and PI3K/mTOR effector pathways is necessary to effectively inhibit NRAS mutant melanoma in vitro and in vivo[J]. Proc Natl Acad Sci U S A, 2013, 110(10): 4015-4020.
doi: 10.1073/pnas.1216013110 |
| [44] |
JURIC D, SORIA J C, SHARMA S, et al. A phase 1b dose-escalation study of BYL719 plus binimetinib (MEK162) in patients with selected advanced solid tumors[J]. J Clin Oncol, 2014, 32(15_suppl): 9051.
doi: 10.1200/jco.2014.32.15_suppl.9051 |
| [45] | ALGAZI A P, ESTEVE-PUIG R, NOSRATI A, et al. Dual MEK/AKT inhibition with trametinib and GSK2141795 does not yield clinical benefit in metastatic NRAS-mutant and wild-type melanoma[J]. Pigment Cell Melanoma Res, 2018, 31(1): 110-114. |
| [46] |
SCHULER M, ZIMMER L, KIM K B, et al. Phase Ⅰb/Ⅱ trial of ribociclib in combination with binimetinib in patients with NRAS-mutant melanoma[J]. Clin Cancer Res, 2022, 28(14): 3002-3010.
doi: 10.1158/1078-0432.CCR-21-3872 |
| [47] | InxMed releases data demonstrating IN10018 therapeutic potential in patients with metastatic melanoma at SMR 2022[R]. SMR, October 17-20, 2022 (Abstract:141). |
| [48] |
KINSEY C G, CAMOLOTTO S A, BOESPFLUG A M, et al. Protective autophagy elicited by RAF→MEK→ERK inhibition suggests a treatment strategy for RAS-driven cancers[J]. Nat Med, 2019, 25(4): 620-627.
doi: 10.1038/s41591-019-0367-9 |
| [49] |
RIBAS A, ALGAZI A, ASCIERTO P A, et al. PD-L1 blockade in combination with inhibition of MAPK oncogenic signaling in patients with advanced melanoma[J]. Nat Commun, 2020, 11(1): 6262.
doi: 10.1038/s41467-020-19810-w pmid: 33288749 |
| [50] |
GOGAS H, DRÉNO B, LARKIN J, et al. Cobimetinib plus atezolizumab in BRAFV600 wild-type melanoma: primary results from the randomized phase Ⅲ IMspire170 study[J]. Ann Oncol, 2021, 32(3): 384-394.
doi: 10.1016/j.annonc.2020.12.004 |
| [51] | GAO X J, XUE D D, CHENG J J, et al. Inhibition of axl promotes the therapeutic effect of targeted inhibition of the PI3K/Akt pathway in NRAS mutant melanoma cells[J]. J Oncol, 2022, 2022: 2946929. |
| [52] |
LI S, WU X, YAN X, et al. Toripalimab plus axitinib in patients with metastatic mucosal melanoma: 3-year survival update and biomarker analysis[J]. J Immunother Cancer, 2022, 10(2): e004036.
doi: 10.1136/jitc-2021-004036 |
| [53] |
PUZANOV I, SOSMAN J, SANTORO A, et al. Phase Ⅰ trial of tivantinib in combination with sorafenib in adult patients with advanced solid tumors[J]. Invest New Drugs, 2015, 33(1): 159-168.
doi: 10.1007/s10637-014-0167-5 |
| [54] |
MAO L, GUO J, ZHU L, et al. A first-in-human, phase 1a dose-escalation study of the selective MEK1/2 inhibitor FCN-159 in patients with advanced NRAS-mutant melanoma[J]. Eur J Cancer, 2022, 175: 125-135.
doi: 10.1016/j.ejca.2022.08.005 |
| [55] |
HAARBERG H E, PARAISO K H, WOOD E, et al. Inhibition of Wee1, AKT, and CDK4 underlies the efficacy of the HSP90 inhibitor XL888 in an in vivo model of NRAS-mutant melanoma[J]. Mol Cancer Ther, 2013, 12(6): 901-912.
doi: 10.1158/1535-7163.MCT-12-1003 |
| [56] |
QIAN L, CHEN K, WANG C H, et al. Targeting NRAS-mutant cancers with the selective STK19 kinase inhibitor chelidonine[J]. Clin Cancer Res, 2020, 26(13): 3408-3419.
doi: 10.1158/1078-0432.CCR-19-2604 |
| [1] | FENG Xinying, WANG Bing, LIU Peifeng. Innovations and challenges in intraperitoneal chemotherapy for peritoneal metastatic carcinoma [J]. China Oncology, 2024, 34(9): 827-837. |
| [2] | CAO Xiaoshan, YANG Beibei, CONG Binbin, LIU Hong. The progress of treatment for brain metastases of triple-negative breast cancer [J]. China Oncology, 2024, 34(8): 777-784. |
| [3] | HUANG Sijie, KANG Xun, LI Wenbin. Clinical research progress of intrathecal therapy in the treatment of leptomeningeal metastasis [J]. China Oncology, 2024, 34(7): 695-701. |
| [4] | LI Xiaohui, ZHAO Jiaxu, PENG Haibao, ZHANG Ye, ZENG Rui, CHI Yudan. Effects of HMGA2 on migration and proliferation of leptomeningeal metastatic melanoma [J]. China Oncology, 2024, 34(4): 389-399. |
| [5] | XU Yonghu, XU Dazhi. Progress and prospects of gastric cancer treatment in the 21st century [J]. China Oncology, 2024, 34(3): 239-249. |
| [6] | CHEN Yifan, LI Ting, WANG Biyun. Research progress of CCR8 in tumor immunotherapy [J]. China Oncology, 2024, 34(3): 299-305. |
| [7] | JIN Yizi, LIN Mingxi, ZENG Cheng, GUO Qing, ZHANG Jian. Research advances in estrogen receptor low positive early breast cancer [J]. China Oncology, 2024, 34(10): 972-978. |
| [8] | LIU Xuerou, YANG Yumei, ZHAO Qian, RONG Xiangyu, LIU Wei, ZHENG Ruijie, PANG Jinlong, LI Xian, LI Shanshan. Research progress on the role of glutamine metabolism-related proteins in tumor metastasis [J]. China Oncology, 2024, 34(1): 97-103. |
| [9] | KANG Yinnan, CHEN Shun, XIE Youcheng, ZHENG Ying, HE Yujing, LI Chuyi, YU Xiaohui. Application and research progress of antibody drug conjugates in HER2 positive advanced gastric cancer [J]. China Oncology, 2023, 33(8): 790-800. |
| [10] | WU Jing, ZHOU Juan, SU Chunxia. Advances in fatty acid metabolism reprogramming of lung cancer [J]. China Oncology, 2023, 33(5): 517-526. |
| [11] | JIANG Jinling, ZHOU Chenfei, WANG Chao, ZHAO Liqin, WU Junwei, ZHANG Jun. Advanced progress in research and diagnosis of gastric cancer in 2022 [J]. China Oncology, 2023, 33(4): 303-314. |
| [12] | YANG Ziyi, LI Panli, GU Bingxin, LIU Cheng, SONG Shaoli, XU Xiaoping. The synthesis of a novel molecular imaging probe 68Ga-DOTA-PDL1P and application in mouse model of melanoma [J]. China Oncology, 2023, 33(4): 354-360. |
| [13] | TIAN Xi, XU Wenhao, ZHU Shuxuan, AIHETAIMUJIANG•Anwaier, SU Jiaqi, YE Shiqi, QU Yuanyuan, SHI Guohai, ZHANG Hailiang, YE Dingwei. Advances in the research, diagnosis and treatment of renal cell carcinoma in 2022 [J]. China Oncology, 2023, 33(3): 191-200. |
| [14] | SU Chunxia, ZHOU Caicun. Important clinical research progress in lung cancer in 2022 [J]. China Oncology, 2023, 33(3): 218-227. |
| [15] | CAO Xiaoshan, CONG Binbin. The research progress of endocrine therapy combined with targeted therapy for triple-positive breast cancer [J]. China Oncology, 2023, 33(3): 288-292. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
沪ICP备12009617
Powered by Beijing Magtech Co. Ltd

