Welcome to China Oncology,
China Oncology ›› 2025, Vol. 35 ›› Issue (2): 237-248.doi: 10.19401/j.cnki.1007-3639.2025.02.011
• Article • Previous Articles Next Articles
LI Yuan1( ), GUO Lingchuan2, YUAN Yong3, ZHENG Qiang1, JIN Yan1, MING Jian4
), GUO Lingchuan2, YUAN Yong3, ZHENG Qiang1, JIN Yan1, MING Jian4
Received:2024-08-30
Revised:2024-11-04
Online:2025-02-28
Published:2025-03-19
Share article
LI Yuan, GUO Lingchuan, YUAN Yong, ZHENG Qiang, JIN Yan, MING Jian. Cost-effectiveness of PD-L1 testing to guide immunotherapy for patients with non-small cell lung cancer in China[J]. China Oncology, 2025, 35(2): 237-248.
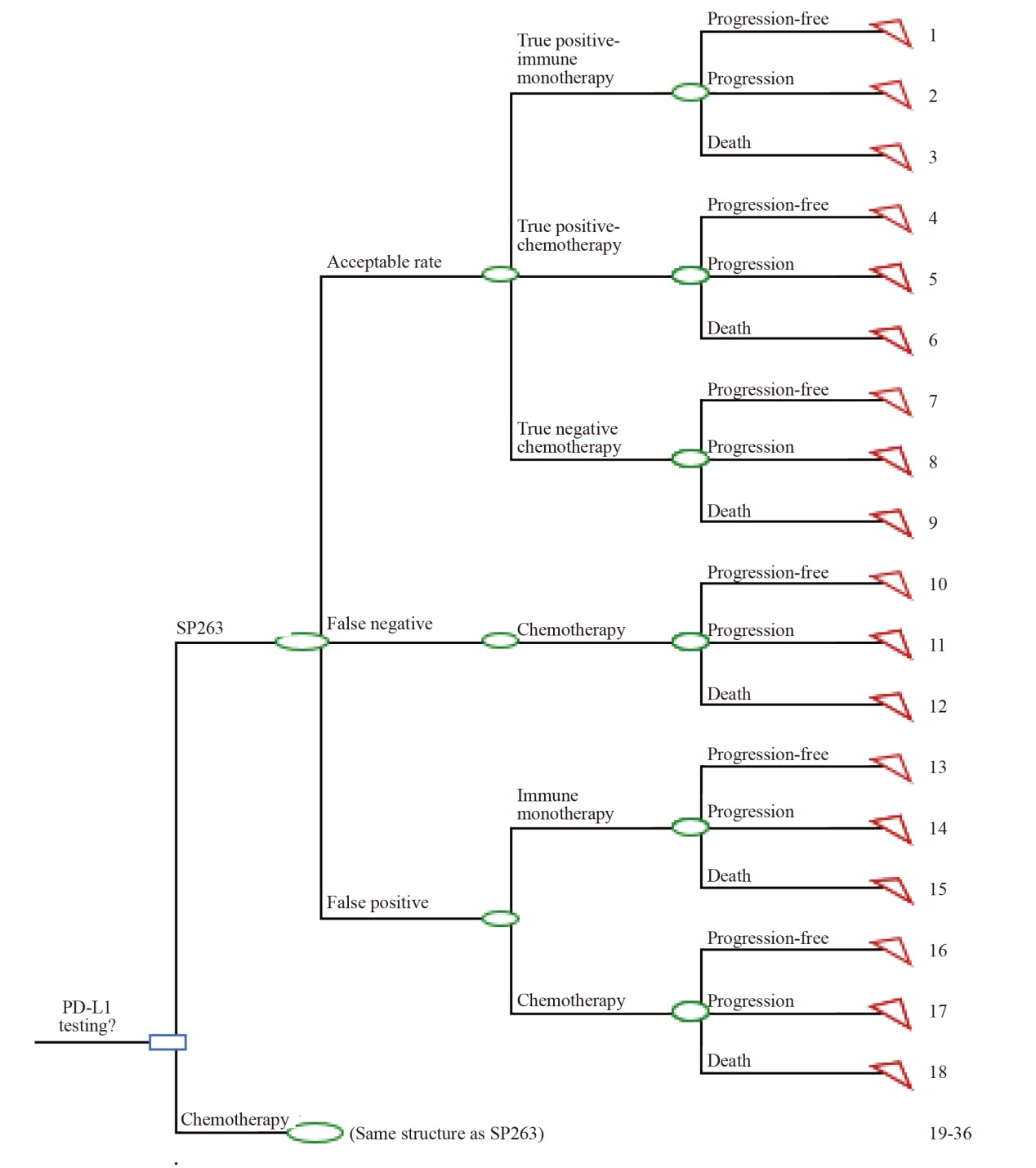
Fig. 1
Decision tree model for PD-L1 testing guided immunotherapy in advanced NSCLC patients Acceptable rate: Including true positive and true negative results, serving as a comprehensive indicator of sensitivity and specificity; Comparator included: 22C3 assay and 22C3 antibody concentrate."
Tab. 1
Summary of model parameters"
| Parameters | Values | Data sources |
|---|---|---|
| Diagnostic performance | ||
| SP263 acceptable rate | 90.55% | NordiQC, PD-L1 (lung) assessment run C1-C5 (2017-2019)[ |
| 22C3 assay acceptable rate | 85.81% | |
| 22C3 antibody concentrate acceptable rate | 75.63% | |
| SP263 false positive, false negative | 1.32%, 8.13% | |
| 22C3 assay false positive, false negative | 1.98%, 12.21% | |
| 22C3 antibody concentrate false positive, false negative | 3.40%, 20.97% | |
| Proportion of patients with positive PD-L1 expression | ||
| Proportion of early to mid-stage NSCLC patients with positive PD-L1 expression (TPS/TC≥1%) | 33.29% | Zheng Q, et al. 2021[ |
| Proportion of advanced NSCLC patients with positive PD-L1 expression (TPS/TC≥50%) | 23.76% | |
| Proportion of drug treatment | ||
| Proportion of early to mid-stage NSCLC patients receiving BSC after positive PD-L1 testing | 60.00% | Expert interviews |
| Proportion of advanced NSCLC patients receiving chemotherapy after positive PD-L1 testing | 19.00% | |
| Proportion of advanced NSCLC patients treated with atezolizumab and pembrolizumab after positive PD-L1 testing (TPS/TC≥50%) | 32.00%, 68.00% | |
| Clinical parameters | ||
| mDFS in early to mid-stage NSCLC patients treated with atezolizumab monotherapy | Not estimable | IMPOWER 010 [ |
| mDFS in early to mid-stage NSCLC patients treated with BSC monotherapy | 35.3 months | IMPOWER 010 |
| mPFS in advanced NSCLC patients treated with atezolizumab monotherapy | 8.2 months | IMPOWER 110 [ |
| mPFS in advanced NSCLC patients treated with chemotherapy | 5.0 months | IMPOWER 110 |
| mPFS in advanced NSCLC patients treated with pembrolizumab monotherapy | 10.3 months | KEYNOTE 024 [ |
| mPFS in advanced NSCLC patients treated with chemotherapy | 6.0 months | KEYNOTE 024 |
| Cost parameters (¥) | ||
| Testing cost | ||
| PD-L1 testing cost | ¥1 450 | Median price charges for medical services by province in China |
| Drug treatment cost | ||
| Atezolizumab price (1 200 mg) | ¥ 32 800 | China Tendering Drugs Database |
| Pembrolizumab price (100 mg) | ¥17 918 | China Tendering Drugs Database |
| BSC cost (3 weeks) | ¥1 887 | Chen P, et al. 2022 [ |
| Cisplatin (10 mg) | ¥10 | China Tendering Drugs Database |
| Carboplatin (50 mg) | ¥67 | China Tendering Drugs Database |
| Pemetrexed (100 mg) | ¥311 | China Tendering Drugs Database |
| Irinotecan (10 mg) | ¥222 | China Tendering Drugs Database |
| Gemcitabine (200 mg) | ¥43 | China Tendering Drugs Database |
| Docetaxel (20 mg) | ¥147 | China Tendering Drugs Database |
| Adverse events administration costs | ||
| Anemia | ¥6 434 | Gu X, et al. 2019[ |
| Fatigue | ¥746 | Wu B, et al. 2012[ |
| Pneumonia | ¥2 500 | Expert interviews |
| Thrombocytopenia | ¥9 693 | Tang Y Q, 2023[ |
| Neutropenia | ¥3 510 | Shi F H, 2021[ |
| Healthcare resource utilization costs | ||
| Complete blood count | ¥10 | Median price charges for medical services by province in China |
| Comprehensive metabolic panel | ¥188 | |
| Chest CT | ¥173 | |
| MRI scan | ¥419 | |
| PET/CT | ¥6 600 | |
| Ultrasound scan | ¥30 | |
| Whole body bone scan | ¥450 | |
| Tumor markers | ¥229 |
Tab. 2
Base case results for early to mid-stage NSCLC patients"
| Testing methods | Cost | Diagnose and treatment rate/% | Incremental cost (Δcost) | Incremental diagnose and treatment rate (Δrate)/% | ICER (Δcost/Δrate) |
|---|---|---|---|---|---|
| SP263 assay | ¥70 841 | 72.47 | |||
| 22C3 assay | ¥70 483 | 68.67 | ¥359 | 3.80 | 9 449 |
| 22C3 antibody concentrate | ¥69 713 | 60.52 | ¥1 129 | 11.94 | 9 449 |
Tab. 3
Base case results for advanced NSCLC patients"
| Testing methods | Cost | Diagnose and treatment rate/% | Incremental cost (Δcost) | Incremental diagnose and treatment rate (Δrate)/% | ICER (Δcost/Δrate) |
|---|---|---|---|---|---|
| SP263 assay | ¥58 306 | 86.46 | |||
| 22C3 assay | ¥61 720 | 81.93 | -¥3 414 | 4.53 | SP263 was superior |
| 22C3 antibody concentrate | ¥61 365 | 72.21 | -¥3 059 | 14.25 | SP263 was superior |
| [1] | ZHENG R S, CHEN R, HAN B F, et al. Cancer incidence and mortality in China, 2022[J]. J Natl Cancer Cent, 2024, 4(1): 47-53. |
| [2] | LIU G B, PEI F, YANG F Q, et al. Role of autophagy and apoptosis in non-small cell lung cancer[J]. Int J Mol Sci, 2017, 18(2): 367. |
| [3] | 中国抗癌协会肿瘤病理专业委员会肺癌协作组、分子病理协作组, 中国抗癌协会肺癌专业委员会, 中国临床肿瘤学会非小细胞肺癌专业委员会. 非小细胞肺癌PD-L1表达临床检测中国专家共识(2023版)[J]. 中华病理学杂志, 2024, 53(2): 121-129. |
| The Lung Cancer Collaboration Group and Molecular Pathology Collaboration Group of the Tumor Pathology Professional Committee of the China Anti-Cancer Association, the Lung Cancer Professional Committee of the Chinese Anti Cancer Association, and the Non-Small Cell Lung Cancer Professional Committee of the Chinese Society of Clinical Oncology. Chinese expert consensus on clinical testing standards of PD-L1 expression for non-small cell lung cancer (2023 version)[J]. Chin J Pathol, 2024, 53(2): 121-129. | |
| [4] | 中国抗癌协会肿瘤病理专业委员会肺癌学组, 中国抗癌协会肺癌专业委员会, PD-L检测共识专家组. 非小细胞肺癌PD-L1免疫组织化学检测规范中国专家共识[J]. 中国肺癌杂志, 2020, 23(9): 733-740. |
| Chinese Anti-Cancer Association, Lung Cancer Study Group of Committee of Oncopathology; Chinese Society of Lung Cancer; Expert Group on PD-L1 Testing Consensus. Chinese expert consensus on standards of PD-L1 immunohistochemistry testing for non-small cell lung cancer[J]. Chin J Lung Cancer, 2020, 23(9): 733-740. | |
| [5] | 中国抗癌协会肿瘤病理专业委员会, 中国临床肿瘤学会肿瘤病理专家委员会, 中国临床肿瘤学会非小细胞肺癌专家委员会. 中国非小细胞肺癌PD-L1表达检测临床病理专家共识[J]. 中华肿瘤杂志, 2020, 42(7): 513-521. |
| China Anti-Cancer Association Tumor Pathology Professional Committee, Chinese Society of Clinical Oncology Tumor Pathology Expert Committee, Chinese Society of Clinical Oncology Non-Small Cell Lung Cancer Expert Committee. Expert consensus on PD-L1 expression testing in non-small-cell lung cancer in China[J]. Chin J Oncol, 2020, 42(7): 513-521. | |
| [6] | 中国临床肿瘤学会(CSCO). 非小细胞肺癌诊疗指南2023[M]. 北京: 人民卫生出版社. |
| Chinese Society of Clinical Oncology (CSCO). Diagnosis and treatment guidelines for non-small cell lung cancer 2023[M]. Beijing: People's Health Publishing House. | |
| [7] | 中华医学会肿瘤学分会, 中华医学会杂志社. 中华医学会肺癌临床诊疗指南(2023版)[J]. 中华医学杂志, 2023, 103(27): 2037-2074. |
| Chinese Medical Association Oncology Branch, Journal of Chinese Medical Association. Chinese Medical Association guideline for clinical diagnosis and treatment of lung cancer (2023 edition)[J]. Natl Med J China, 2023, 103(27): 2037-2074. | |
| [8] | NordiQC, PD-L1(lung) assessment run C1-C5(2017-2019)[EB/OL]. https://www.nordiqc.org/epitope.php?id=107. |
| [9] | ZHENG Q, HUANG Y, ZENG X, et al. Clinicopathological and molecular characteristics associated with PD-L1 expression in non-small cell lung cancer: a large-scale, multi-center, real-world study in China[J]. J Cancer Res Clin Oncol, 2021, 147(5): 1547-1556. |
| [10] | FELIP E, ALTORKI N, ZHOU C, et al. Overall survival with adjuvant atezolizumab after chemotherapy in resected stage Ⅱ-ⅢA non-small cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase 3 trial[J]. Ann Oncol, 2023, 34(10): 907-919. |
| [11] | HERBST R S, GIACCONE G, DE MARINIS F, et al. Atezolizumab for first-line treatment of PD-L1-selected patients with NSCLC[J]. N Engl J Med, 2020, 383(14): 1328-1339. |
| [12] | RECK M, RODRÍGUEZ-ABREU D, ROBINSON A G, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer[J]. N Engl J Med, 2016, 375(19): 1823-1833. |
| [13] | CHEN P, YANG Q, LI Y F, et al. Cost-effectiveness analysis of adjuvant therapy with atezolizumab in Chinese patients with stage ⅠB-ⅢA resectable NSCLC after adjuvant chemotherapy[J]. Front Oncol, 2022, 12: 894656. |
| [14] |
GU X H, ZHANG Q, CHU Y B, et al. Cost-effectiveness of afatinib, gefitinib, erlotinib and pemetrexed-based chemotherapy as first-line treatments for advanced non-small cell lung cancer in China[J]. Lung Cancer, 2019, 127: 84-89.
doi: S0169-5002(18)30668-8 pmid: 30642557 |
| [15] | WU B, DONG B J, XU Y J, et al. Economic evaluation of first-line treatments for metastatic renal cell carcinoma: a cost-effectiveness analysis in a health resource-limited setting[J]. PLoS One, 2012, 7(3): e32530. |
| [16] | 汤雅倩, 赵明烨, 唐文熙. 信迪利单抗对比卡瑞利珠单抗一线治疗晚期非鳞状非小细胞肺癌的经济学评价[J]. 卫生经济研究, 2023, 40(2): 34-40. |
| TANG Y Q, ZHAO M Y, TANG W X. Pharmacoeconomic evaluation of sintilimab versus camrelizumab in the first-line treatment of patients with non-squamous advanced non-small cell lung cancer in China[J]. Health Econ Res, 2023, 40(2): 34-40. | |
| [17] | 石丰豪, 金敏, 王子婧, 等. 卡瑞利珠单抗对比化疗方案二线治疗晚期或转移性食管鳞状细胞癌的成本效用分析[J]. 中国卫生经济, 2021, 40(12): 73-77. |
| SHI F H, JIN M, WANG Z J, et al. Cost-utility analysis of camrelizumab versus chemotherapy regimens for second-line treatment of advanced or met-astatic esophageal squamous cell carcinoma[J]. Chin Health Econ, 2021, 40(12): 73-77. | |
| [18] | 中华慈善总会泰圣奇慈善援助项目[EB/OL]. https://tpap.yao2000.com.cn/flow.html. |
| China Charity Federation Tai Shengqi Charity assistance project[EB/OL]. https://tpap.yao2000.com.cn/flow.html. | |
| [19] | 中国初级卫生保健基金会生命之钥-肿瘤免疫治疗患者援助项目. https://smzy.ilvzhou.com/index.php?m=content&c=index&a=lists&catid=31. |
| China Primary Health Care Foundation's key to life-tumor immunotherapy patient assistance project[EB/OL]. https://smzy.ilvzhou.com/index.php?m=content&c=index&a=lists&catid=31. | |
| [20] | HURWITZ J T, VAFFIS S, GRIZZLE A J, et al. Cost-effectiveness of PD-L1 testing in non-small cell lung cancer (NSCLC) using in vitro diagnostic (IVD) versus laboratory-developed test (LDT)[J]. Oncol Ther, 2022, 10(2): 391-409. |
| [21] | SHIMABUKURO HO R, MIOTI SEBASTIÃO M, VENEZIAN DE CARVALHO J P, et al. Cost-effectiveness analysis of the SP142 versus 22C3 PD-L1 assays in the treatment of atezolizumab plus nab-paclitaxel for patients with advanced triple negative breast cancer in the Brazilian private healthcare system[J]. J Med Econ, 2020, 23(11): 1340-1344. |
| [22] | WAN N, ZHANG T T, HUA S H, et al. Cost-effectiveness analysis of pembrolizumab plus chemotherapy with PD-L1 test for the first-line treatment of NSCLC[J]. Cancer Med, 2020, 9(5): 1683-1693. |
| [23] | HUANG M, LOU Y Y, PELLISSIER J, et al. Cost effectiveness of pembrolizumab vs standard-of-care chemotherapy as first-line treatment for metastatic NSCLC that expresses high levels of PD-L1 in the United States[J]. PharmacoEconomics, 2017, 35(8): 831-844. |
| [24] |
DING D, HU H B, LIAO M T, et al. Cost-effectiveness analysis of atezolizumab plus chemotherapy in the first-line treatment of metastatic non-squamous non-small cell lung cancer[J]. Adv Ther, 2020, 37(5): 2116-2126.
doi: 10.1007/s12325-020-01292-3 pmid: 32193809 |
| [25] | 郭雪晶, 曹赫, 周建娅, 等. PD-L1检测方法在非小细胞肺癌的研究进展[J]. 中国肺癌杂志, 2019, 22(1): 40-44. |
| GUO X J, CAO H, ZHOU J Y, et al. Progress on the study of PD-L1 detection methods in non-small cell lung cancer[J]. Chin J Lung Cancer, 2019, 22(1): 40-44. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
沪ICP备12009617
Powered by Beijing Magtech Co. Ltd
