Welcome to China Oncology,
China Oncology ›› 2024, Vol. 34 ›› Issue (10): 944-956.doi: 10.19401/j.cnki.1007-3639.2024.10.004
• Article • Previous Articles Next Articles
TAN Xiaolang1( ), YAO Sha2, WANG Guihua1, PENG Luogen1(
), YAO Sha2, WANG Guihua1, PENG Luogen1( )
)
Received:2024-05-27
Revised:2024-09-10
Online:2024-10-30
Published:2024-11-20
Contact:
PENG Luogen
Share article
CLC Number:
TAN Xiaolang, YAO Sha, WANG Guihua, PENG Luogen. Research on uPAR promoting proliferation, migration, and chemoresistance of pancreatic cancer by inhibiting autophagy via MAPK signaling[J]. China Oncology, 2024, 34(10): 944-956.
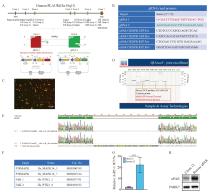
Fig. 1
uPAR knockout strategy and sequencing gene and uPAR re-expression A: Schematic representation of the uPAR knockout strategy using CRISPR/Cas9. B: List of gRNAs for uPAR and PCR primers used to detect gene knockout. C: Fluorescence-activated cell sorting (FACS) analysis of GFP/RFP double-positive cells. D: PCR screening for gRNA target sites and potential deletions. E: Sanger sequencing of mutations at the gRNA target sites. F: Sequences of siRNA oligonucleotide templates used in this study. G: Re-expression of uPAR protein levels measured by ELISA (n=3, ***: P<0.01, Student’s t-test, two-sided). H: Immunoblot analysis showing de novo expression of uPAR in clone 12 and rescued uPAR cells."
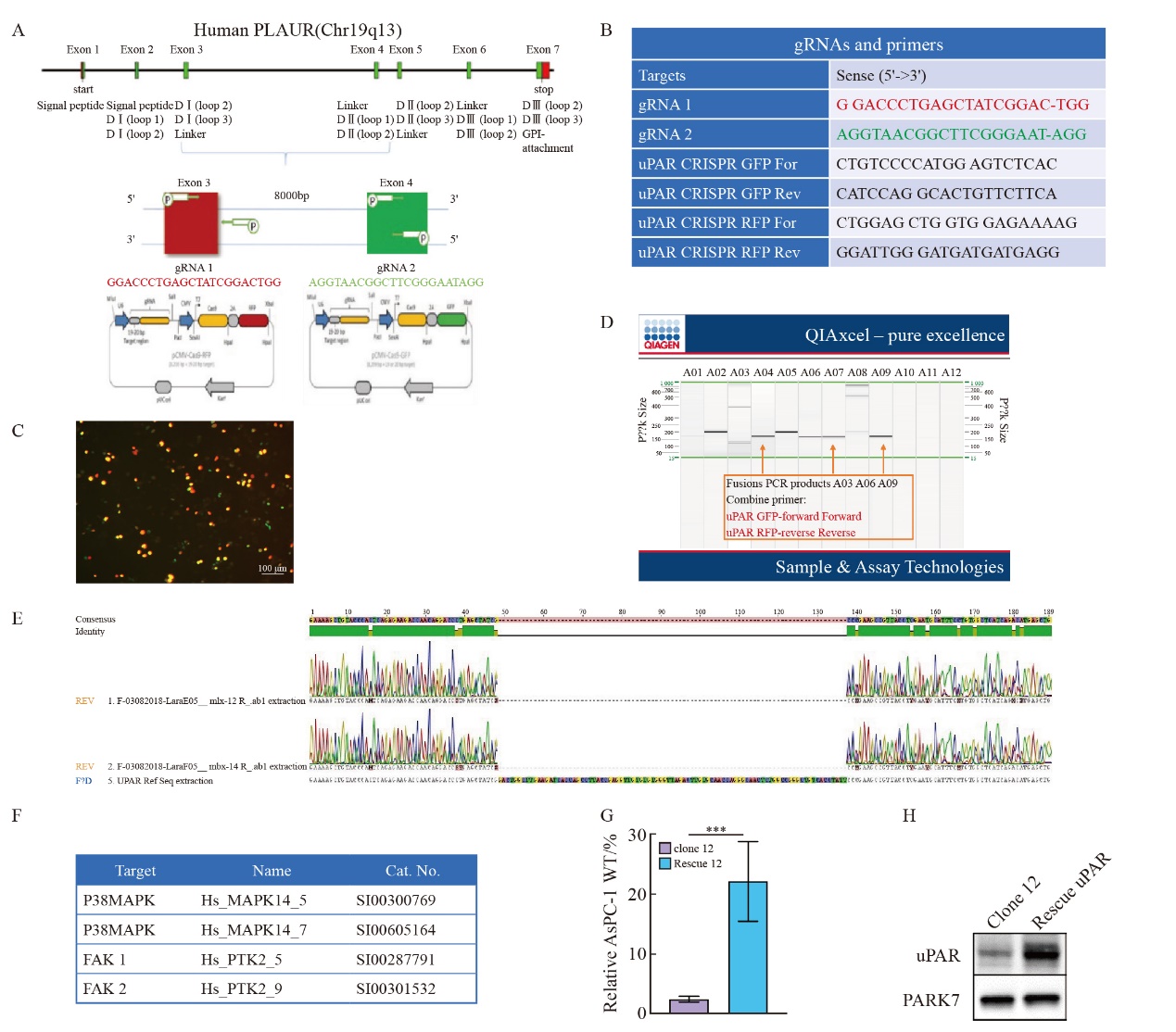
Fig. 2
CRISPR/Cas9-mediated uPAR knockout in AsPC-1 cells A: Immunoblot analysis showing uPAR protein levels in the pancreatic cell lines AsPC-1, CAPAN-1, CAPAN-2, MIA PaCa-2, PANC-1, and PaTu8988T. B: ELISA quantification of uPAR protein levels in 6 human pancreatic cell lines. C: Immunoblot analysis comparing uPAR levels between AsPC-1 uPAR knockout (KO) and wild-type (WT) cells. D: ELISA results showing uPAR levels in AsPC-1 uPAR KO cells compared to WT cells. At least three biological replicates were performed. GAPDH was used as a loading control. ***: P<0.001, multiple comparison tests were used to assess differences between groups."
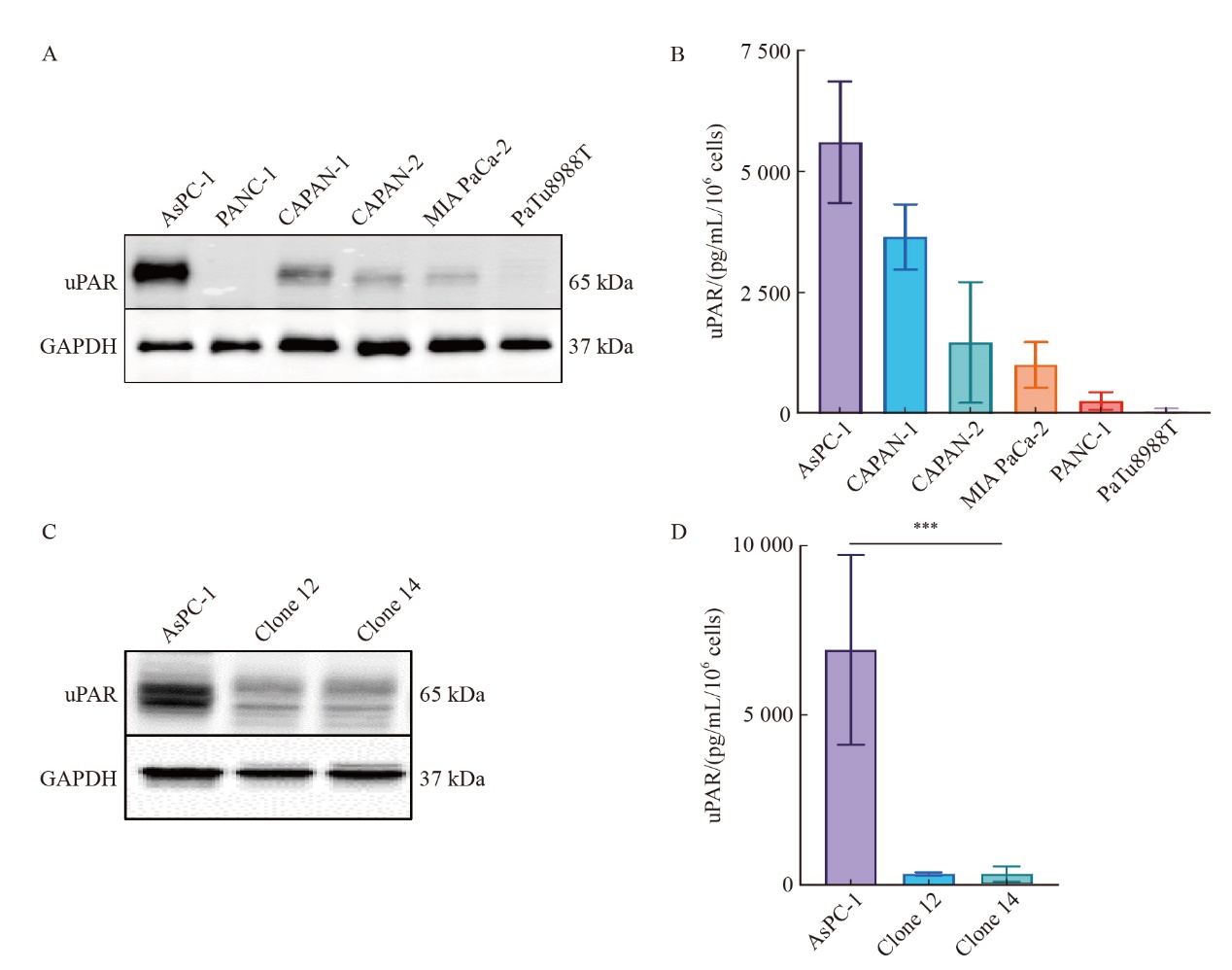
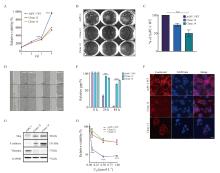
Fig. 3
uPAR knockout inhibits pancreatic cancer cell growth and migration, and enhances resistance to gemcitabine A: Growth curves over 4 days showing significantly reduced proliferation rates in AsPC-1 uPAR KO clones compared to WT controls. B: Representative colony formation assay images in methylcellulose comparing uPAR KO and WT cells. C: Quantitative analysis showing a significant reduction in clonogenicity in uPAR KO cells compared to WT cells. D-E: Reduced migratory capacity of uPAR KO cells compared to WT cells. F: Immunofluorescence staining showing fewer stress fibers in uPAR KO cells compared to WT cells (×400, red: phalloidin, blue: DAPI). G: Immunoblot analysis of epithelial and mesenchymal markers indicating Mesenchymal-to-epithelial transition (MET) in uPAR KO cells. H: Increased resistance to gemcitabine in uPAR KO cells (0.1, 0.5, 1 µmol/L, 72 h). At least three biological replicates were performed. GAPDH or PARK7 was used as a loading control. ***: P<0.001, multiple comparison tests were used to assess differences between groups."
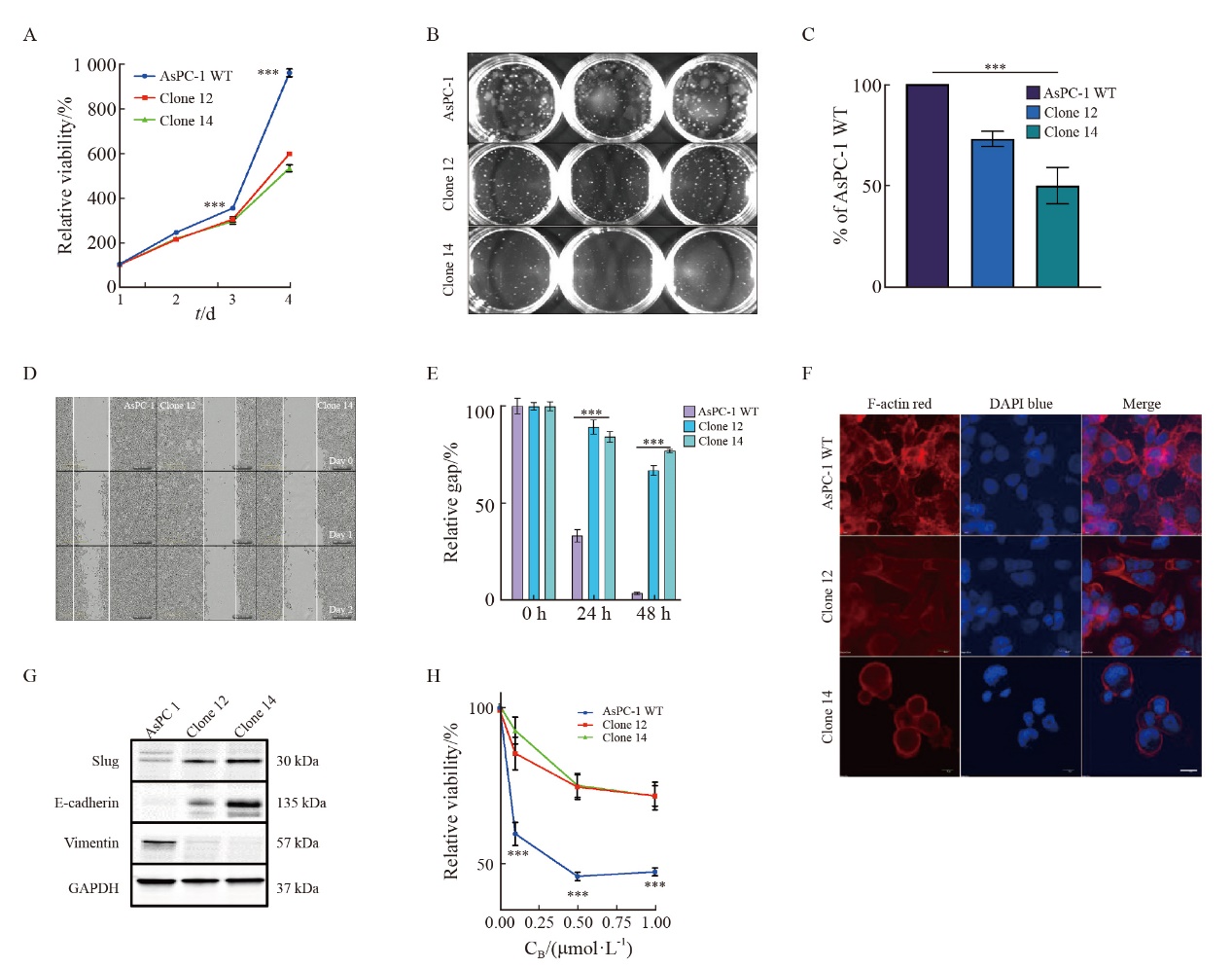
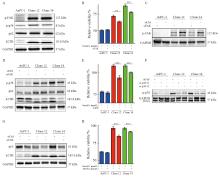
Fig. 4
uPAR regulates FAK and p38MAPK activity and enhaces gemCitabine resensitivity of AsPC-1 uPAR knockout cells A: Immunoblot analysis showing increased phosphorylation of FAK and p38, along with elevated levels of LC3B, while p62 levels remain unchanged in uPAR knockout (KO) cells. B-D: Restoration of gemCitabine sensitivity (B) and wild-type (WT) signaling phenotype (D) in AsPC-1 uPAR KO cells after (C) FAK siRNA knockdown. E-F: Gemcitabine response following p38MAPK knockdown (F) using two siRNAs in AsPC-1 uPAR KO cells. G: Re-establishment of the WT signaling phenotype in uPAR KO cells following p38MAPK siRNA knockdown. H: Combined treatment with gemcitabine (0.1 µmol/L) and p38 inhibitor JX401 (3 µmol/L) for 72 h in uPAR KO cells. GAPDH was used as a loading control. At least three biological replicates. **: P <0.01, ***: P<0.001, Student’s t test, two-sided."
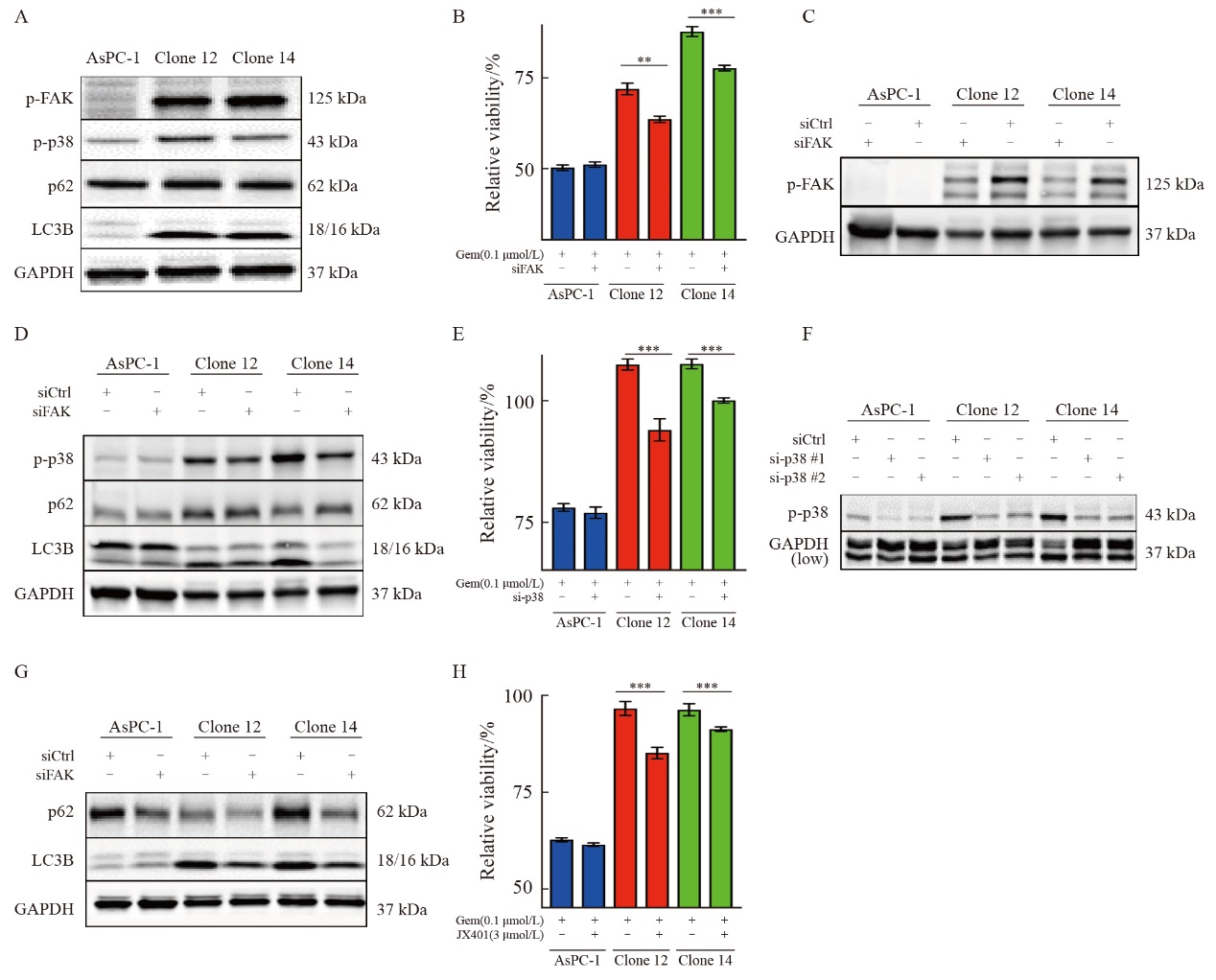
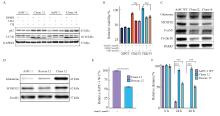
Fig.5
Inhibition of autophagy or uPAR re-expression restores gemcitabine sensitivity in uPAR knockout cells A: Immunoblot analysis of autophagy markers p62 and LC3B following treatment with 3-MA (5 µmol/L) or chloroquine (CQ, 5 µmol/L). B: GemCitabine sensitivity after 72 hours of treatment with 3-MA (5 µmol/L) or CQ (5 µmol/L). C: Immunoblot analysis showing altered expression of glycolytic enzymes (GLUL, MTHFD2) and cell cycle regulators (FOXM1, CCNB1), suggesting dormancy in uPAR knockout (KO) cells. D: Re-expression of uPAR in clone 12 cells restored p-p38MAPK and p62 levels to wild-type (WT) levels. E: Re-expression of uPAR restored gemcitabine sensitivity in clone 12 cells (0.1 µmol/L). F: Re-expression of uPAR significantly enhanced migratory capacity in clone 12 cells. GAPDH or PARK7 was used as a loading control. At least three biological replicates were performed. ***: P<0.001, Student’s t-test, two-sided."
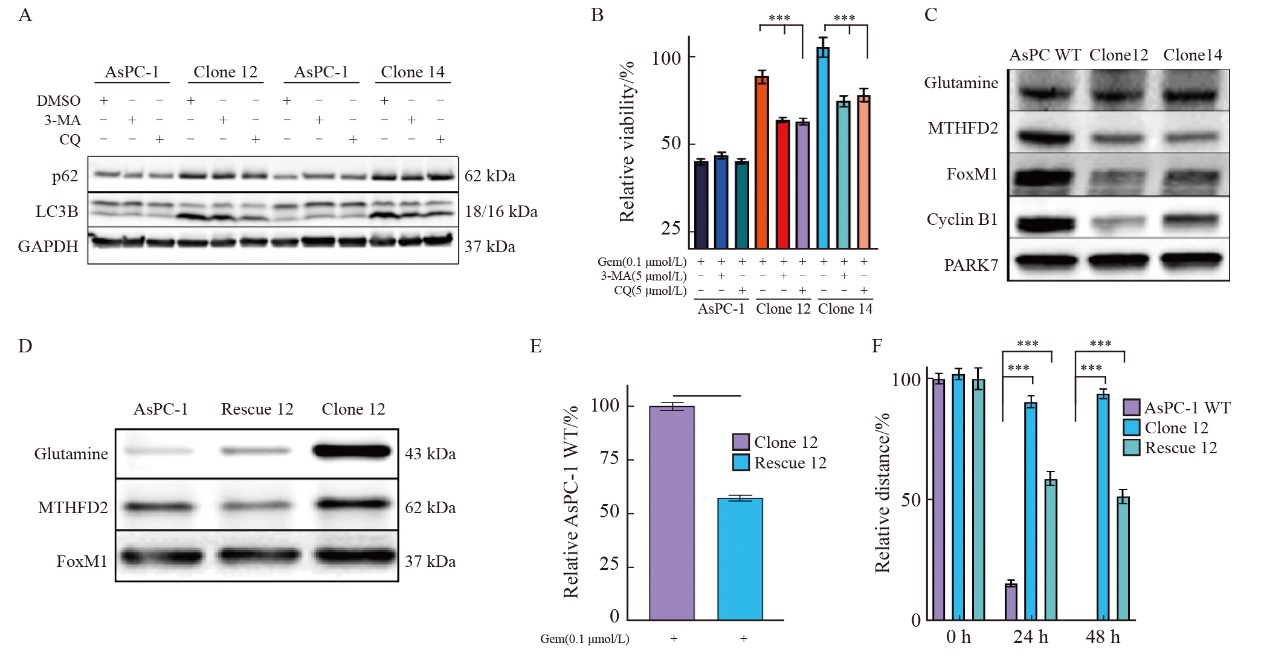

Fig. 6
uPAR as a predictive biomarker for gemcitabine sensitivity in PDAC-derived organoids A: Representative bright-field images of patient-derived PDAC organoids (PDOs) showing three distinct morphologies: organoids with varying degrees of budding (top), solid organoids (center), and hollow organoids (bottom). Scale bar: 200 µm. B: Dose-response curves for gemcitabine in PDOs cultures (n=8) treated with gemcitabine (0.01-10.00 µmol/L). C: IC50 values for PDOs cultures treated with gemcitabine. D: Immunoblot analysis of uPAR and LC3B in protein extracts from eight PDOs, with PARK7 as a loading control. E: Pearson correlation analysis showing a significant inverse correlation between uPAR levels and gemcitabine sensitivity (IC50) in PDOs (r2=0.66, P=0.026 2). At least three biological replicates were performed. *: P<0.05, compared with each other; **: P<0.01, compared with each other; Student’s t test, two-sided. GEM: Gemcitabine."
| [1] |
CONROY T, PFEIFFER P, VILGRAIN V, et al. Pancreatic cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up[J]. Ann Oncol, 2023, 34(11): 987-1002.
doi: 10.1016/j.annonc.2023.08.009 pmid: 37678671 |
| [2] | LIN W, NOEL P, BORAZANCI E H, et al. Single-cell transcriptome analysis of tumor and stromal compartments of pancreatic ductal adenocarcinoma primary tumors and metastatic lesions[J]. Genome Med, 2020, 12(1): 80. |
| [3] |
PATIL S, STEUBER B, KOPP W, et al. EZH2 regulates pancreatic cancer subtype identity and tumor progression via transcriptional repression of GATA6[J]. Cancer Res, 2020, 80(21): 4620-4632.
doi: 10.1158/0008-5472.CAN-20-0672 pmid: 32907838 |
| [4] |
NOLL E M, EISEN C, STENZINGER A, et al. CYP3A5 mediates basal and acquired therapy resistance in different subtypes of pancreatic ductal adenocarcinoma[J]. Nat Med, 2016, 22(3): 278-287.
doi: 10.1038/nm.4038 pmid: 26855150 |
| [5] | NEVALA-PLAGEMANN C, HIDALGO M, GARRIDO-LAGUNA I. From state-of-the-art treatments to novel therapies for advanced-stage pancreatic cancer[J]. Nat Rev Clin Oncol, 2020, 17(2): 108-123. |
| [6] |
MIZRAHI J D, SURANA R, VALLE J W, et al. Pancreatic cancer[J]. Lancet, 2020, 395(10242): 2008-2020.
doi: S0140-6736(20)30974-0 pmid: 32593337 |
| [7] | KUMAR A A, BUCKLEY B J, RANSON M. The urokinase plasminogen activation system in pancreatic cancer: prospective diagnostic and therapeutic targets[J]. Biomolecules, 2022, 12(2): 152. |
| [8] |
ARONEN A, AITTONIEMI J, HUTTUNEN R, et al. P-suPAR may reflect the inflammatory response after pancreatic surgery[J]. Pancreatology, 2024, 24(1): 146-151.
doi: 10.1016/j.pan.2023.11.006 pmid: 38000982 |
| [9] | ZHAI B T, TIAN H, SUN J, et al. Urokinase-type plasminogen activator receptor (uPAR) as a therapeutic target in cancer[J]. J Transl Med, 2022, 20(1): 135. |
| [10] | PESCE N A, PLASTINO F, REYES-GOYA C, et al. Mitigation of human iris angiogenesis through uPAR/LRP-1 interaction antagonism in an organotypic exvivo model[J]. FASEB J, 2024, 38(5): e23533. |
| [11] | SMITH H W, MARSHALL C J. Regulation of cell signalling by uPAR[J]. Nat Rev Mol Cell Biol, 2010, 11(1): 23-36. |
| [12] |
FERRARIS G M S, SCHULTE C, BUTTIGLIONE V, et al. The interaction between uPAR and vitronectin triggers ligand-independent adhesion signalling by integrins[J]. EMBO J, 2014, 33(21): 2458-2472.
doi: 10.15252/embj.201387611 pmid: 25168639 |
| [13] | TAN X M, YAN Y H, SONG B, et al. Focal adhesion kinase: from biological functions to therapeutic strategies[J]. Exp Hematol Oncol, 2023, 12(1): 83. |
| [14] |
ZHENG Y H, XIA Y, HAWKE D, et al. FAK phosphorylation by ERK primes ras-induced tyrosine dephosphorylation of FAK mediated by PIN1 and PTP-PEST[J]. Mol Cell, 2009, 35(1): 11-25.
doi: 10.1016/j.molcel.2009.06.013 pmid: 19595712 |
| [15] |
ZHENG Y H, LU Z M. Paradoxical roles of FAK in tumor cell migration and metastasis[J]. Cell Cycle, 2009, 8(21): 3474-3479.
pmid: 19829089 |
| [16] |
WU H W, LIANG Z Y, SHI X H, et al. Intrinsic chemoresistance to gemcitabine is associated with constitutive and laminin-induced phosphorylation of FAK in pancreatic cancer cell lines[J]. Mol Cancer, 2009, 8: 125.
doi: 10.1186/1476-4598-8-125 pmid: 20021699 |
| [17] | JI C B, ZHANG M L, HU J J, et al. The kinase activity of integrin-linked kinase regulates cellular senescence in gastric cancer[J]. Cell Death Dis, 2022, 13(7): 577. |
| [18] | ZHOU Y H, XIE Y, LUO Y Z, et al. SNAI2 enhances HPV-negative cervical cancer cell dormancy by modulating u-PAR expression and the activity of the ERK/p38 signaling pathway in vitro[J]. Oncol Rep, 2024, 52(2): 104. |
| [19] | HILDENBRAND R, ALLGAYER H, MARX A, et al. Modulators of the urokinase-type plasminogen activation system for cancer[J]. Expert Opin Investig Drugs, 2010, 19(5): 641-652. |
| [20] | YAO S, PENG L G, ELAKAD O, et al. One carbon metabolism in human lung cancer[J]. Transl Lung Cancer Res, 2021, 10(6): 2523-2538. |
| [21] | BOHNENBERGER H, KADERALI L, STRÖBEL P, et al. Comparative proteomics reveals a diagnostic signature for pulmonary head-and-neck cancermetastasis[J]. EMBO Mol Med, 2018, 10(9): e8428. |
| [22] | ELAKAD O, HÄUPL B, LABITZKY V, et al. Activation of CD44/PAK1/AKT signaling promotes resistance to FGFR1 inhibition in squamous-cell lung cancer[J]. NPJ Precis Oncol, 2022, 6(1): 52. |
| [23] | PENG L G, LI Y C, YAO S, et al. Urokinase-type plasminogen activator receptor (uPAR) cooperates with mutated KRAS in regulating cellular plasticity and gemcitabine response in pancreatic adenocarcinomas[J]. Cancers, 2023, 15(5): 1587. |
| [24] | WIŚNIEWSKI J R, MANN M. A proteomics approach to the protein normalization problem: Selection of unvarying proteins for MS-based proteomics and Western blot[J]. J Proteome Res, 2016, 15(7): 2321-2326. |
| [25] | SHMAKOVA A A, KLIMOVICH P S, RYSENKOVA K D, et al. Urokinase receptor uPAR downregulation in neuroblastoma leads to dormancy, chemoresistance and metastasis[J]. Cancers, 2022, 14(4): 994. |
| [26] | SUGIOKA K, NISHIDA T, KODAMA-TAKAHASHI A, et al. Urokinase-type plasminogen activator negatively regulates α-smooth muscle actin expression via Endo180 and the uPA receptor in corneal fibroblasts[J]. Am J Physiol Cell Physiol, 2022, 323(1): C104-C115. |
| [27] |
LOGAN R, JEFFERS A, QIN W Y, et al. TGF-β regulation of the uPA/uPAR axis modulates mesothelial-mesenchymal transition (MesoMT)[J]. Sci Rep, 2021, 11: 21210.
doi: 10.1038/s41598-021-99520-5 pmid: 34707211 |
| [28] | AGUIRRE-GHISO J A, LIU D, MIGNATTI A, et al. Urokinase receptor and fibronectin regulate the ERK (MAPK) to p38 (MAPK) activity ratios that determine carcinoma cell proliferation or dormancy in vivo[J]. Mol Biol Cell, 2001, 12(4): 863-879. |
| [29] |
LEVY J M M, TOWERS C G, THORBURN A. Targeting autophagy in cancer[J]. Nat Rev Cancer, 2017, 17(9): 528-542.
doi: 10.1038/nrc.2017.53 pmid: 28751651 |
| [30] |
BRYANT K L, STALNECKER C A, ZEITOUNI D, et al. Combination of ERK and autophagy inhibition as a treatment approach for pancreatic cancer[J]. Nat Med, 2019, 25(4): 628-640.
doi: 10.1038/s41591-019-0368-8 pmid: 30833752 |
| [31] | LA BELLE FLYNN A, CALHOUN B C, SHARMA A, et al. Autophagy inhibition elicits emergence from metastatic dormancy by inducing and stabilizing Pfkfb3 expression[J]. Nat Commun, 2019, 10(1): 3668. |
| [32] | BOJ S F, HWANG C I, BAKER L A, et al. Organoid models of human and mouse ductal pancreatic cancer[J]. Cell, 2015, 160(1/2): 324-338. |
| [33] | TOKUMO K, MASUDA T, NAKASHIMA T, et al. Association between plasminogen activator inhibitor-1 and osimertinib tolerance in EGFR-mutated lung cancer via epithelial-mesenchymal transition[J]. Cancers, 2023, 15(4): 1092. |
| [34] |
ZHANG T, WANG B F, SU F, et al. TCF7L2 promotes anoikis resistance and metastasis of gastric cancer by transcriptionally activating PLAUR[J]. Int J Biol Sci, 2022, 18(11): 4560-4577.
doi: 10.7150/ijbs.69933 pmid: 35864968 |
| [35] |
HILDENBRAND R, NIEDERGETHMANN M, MARX A, et al. Amplification of the urokinase-type plasminogen activator receptor (uPAR) gene in ductal pancreatic carcinomas identifies a clinically high-risk group[J]. Am J Pathol, 2009, 174(6): 2246-2253.
doi: 10.2353/ajpath.2009.080785 pmid: 19435784 |
| [36] |
HENKE E, NANDIGAMA R, ERGÜN S. Extracellular matrix in the tumor microenvironment and its impact on cancer therapy[J]. Front Mol Biosci, 2019, 6: 160.
doi: 10.3389/fmolb.2019.00160 pmid: 32118030 |
| [37] | HUANG J Y, LIN Y C, CHEN H M, et al. Adenine combined with cisplatin promotes anticancer activity against hepatocellular cancer cells through AMPK-mediated p53/p21 and p38 MAPK cascades[J]. Pharmaceuticals, 2022, 15(7): 795. |
| [38] | YAMAMOTO K, VENIDA A, YANO J, et al. Autophagy promotes immune evasion of pancreatic cancer by degrading MHC-I[J]. Nature, 2020, 581(7806): 100-105. |
| [39] |
GANDHARI M, ARENS N, MAJETY M, et al. Urokinase-type plasminogen activator induces proliferation in breast cancer cells[J]. Int J Oncol, 2006, 28(6): 1463-1470.
pmid: 16685447 |
| [40] |
GONIAS S L, HU J J. Urokinase receptor and resistance to targeted anticancer agents[J]. Front Pharmacol, 2015, 6: 154.
doi: 10.3389/fphar.2015.00154 pmid: 26283964 |
| [41] | SHIBUE T, WEINBERG R A. EMT, CSCs, and drug resistance: the mechanistic link and clinical implications[J]. Nat Rev Clin Oncol, 2017, 14(10): 611-629. |
| [42] | MEHNERT J M, MITCHELL T C, HUANG A C, et al. BAMM (BRAF autophagy and MEK inhibition in melanoma): a phase Ⅰ/Ⅱ trial of dabrafenib, trametinib, and hydroxychloroquine in advanced BRAFV600-mutant melanoma[J]. Clin Cancer Res, 2022, 28(6): 1098-1106. |
| [43] | JAIN V, HARPER S L, VERSACE A M, et al. Targeting UGCG overcomes resistance to lysosomal autophagy inhibition[J]. Cancer Discov, 2023, 13(2): 454-473. |
| [1] | WANG Ting, QIN Yi, XU Xiaowu, YU Xianjun. New advances in basic research, clinical diagnosis and treatment of pancreatic cancer in 2024 [J]. China Oncology, 2025, 35(1): 1-11. |
| [2] | XIAO Yi, WU Ming, YAO Gang. Research progress and future perspectives of tumor organoid [J]. China Oncology, 2024, 34(8): 763-776. |
| [3] | CHEN Hong, CAO Zhiyun. Recent progress in the construction and application of patient-derived pancreatic cancer organoid models [J]. China Oncology, 2024, 34(6): 590-597. |
| [4] | WU Hongji, WANG Haixia, WANG Ling, LUO Xiaogang, ZOU Dongling. Application progress and challenges of artificial intelligence in organoid research [J]. China Oncology, 2024, 34(2): 210-219. |
| [5] | REN Jiaqiang, WU Shuai, SU Tong, LI Jie, HAN Liang, WU Zheng. An exploratory study of INPP4B, a biomarker of gemcitabine chemoresistance in pancreatic cancer [J]. China Oncology, 2024, 34(12): 1090-1099. |
| [6] | JIANG Yuanyuan, WEI Wenfei, WU Jingya, LI Huawen. Application of organoids in drug screening of gynecological malignant tumors [J]. China Oncology, 2024, 34(11): 1053-1060. |
| [7] | LI Tianjiao, YE Longyun, JIN Kaizhou, WU Weiding, YU Xianjun. Advances in basic research, clinical diagnosis and treatment of pancreatic cancer in 2023 [J]. China Oncology, 2024, 34(1): 1-12. |
| [8] | ZENG Cheng, ZHANG Jian. Leading research progress and prospect of antibody-drug conjugate in pancreatic cancer in 2022 [J]. China Oncology, 2023, 33(3): 235-240. |
| [9] | FU Qingsheng, JIN Lei, ZHANG Xudong, XU Yingchen, ZHU Chunfu, QIN Xihu, WU Baoqiang. Effect of tRF-Pro-CGG on the biological behavior of mouse pancreatic cancer cells and its molecular mechanism [J]. China Oncology, 2023, 33(3): 241-249. |
| [10] | LU Yu, XI Yumeng, HE Xiaoming, YANG Shaokun, ZHANG Jia, WANG Lei, HE Chaoxing, XIANG Bai. Advances in the application of co-culture strategies in organoids [J]. China Oncology, 2023, 33(3): 293-302. |
| [11] | LENG Jie, QIU Guochun, ZHANG Bo, PU Yan. Mechanism of breast cancer centrosome regulatory protein SEC23B on tumor invasion and metastasis [J]. China Oncology, 2023, 33(2): 152-161. |
| [12] | YUE Ming, WANG Liwei, CUI Jiujie. Research progress on the mechanism of organ-specific lung metastasis in pancreatic cancer [J]. China Oncology, 2023, 33(11): 1026-1031. |
| [13] | JIA Yuming, YE Zeng, DENG Yanli, LI Shengchao, ZHANG Zhilei, WANG Chao, XU Xiaowu, QIN Yi, PENG Li. The research on FBW7 gene enhances antitumor effect of paclitaxel on pancreatic cancer through GSDME-mediated pyroptosis [J]. China Oncology, 2023, 33(10): 889-897. |
| [14] | WANG Xu, CHENG He, LIU Chen, YU Xianjun. New progress in basic research, clinical diagnosis and treatment of pancreatic cancer in 2022 [J]. China Oncology, 2023, 33(1): 1-13. |
| [15] | ZHUANG Han, LING Chifang, WANG Jiazhou, HAN Xu, JIANG Rui, HU Weigang. Radiation therapy in locally advanced pancreatic cancer with 75 Gy simultaneous integrated boost: a dosimetric feasibility study [J]. China Oncology, 2023, 33(1): 54-60. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
沪ICP备12009617
Powered by Beijing Magtech Co. Ltd
