Welcome to China Oncology,
China Oncology ›› 2024, Vol. 34 ›› Issue (9): 857-872.doi: 10.19401/j.cnki.1007-3639.2024.09.006
• Article • Previous Articles Next Articles
HUANG Haozhe1( ), CHEN Hong2(
), CHEN Hong2( ), ZHENG Dezhong3, CHEN Chao1, WANG Ying1, XU Lichao1, WANG Yaohui1, HE Xinhong1, YANG Yuanyuan3, LI Wentao1(
), ZHENG Dezhong3, CHEN Chao1, WANG Ying1, XU Lichao1, WANG Yaohui1, HE Xinhong1, YANG Yuanyuan3, LI Wentao1( )
)
Received:2024-05-07
Revised:2024-06-13
Online:2024-09-30
Published:2024-10-11
Contact:
LI Wentao
Share article
CLC Number:
HUANG Haozhe, CHEN Hong, ZHENG Dezhong, CHEN Chao, WANG Ying, XU Lichao, WANG Yaohui, HE Xinhong, YANG Yuanyuan, LI Wentao. A CT-based radiomics nomogram for predicting local tumor progression of colorectal cancer lung metastases treated with radiofrequency ablation[J]. China Oncology, 2024, 34(9): 857-872.
Tab. 1
Characteristics of CRC patients with lung metastases"
| Characteristic | Training dataset (N=323) | Test dataset (N=78) | P value | Total (N=401) |
|---|---|---|---|---|
| Pre-RFA clinical characteristics | ||||
| Gender n | 0.947 | |||
| Male | 185 | 45 | 230 | |
| Female | 138 | 33 | 171 | |
| Age/year M (Q1, Q3) | 57 (50, 65) | 61 (52, 66.25) | 0.120 | |
| Initial tumor location n | 0.166 | |||
| Rectum | 224 | 44 | 268 | |
| Sigmoid-left colon | 40 | 15 | 55 | |
| Transverse-right colon | 57 | 18 | 75 | |
| Caecum | 2 | 1 | 3 | |
| Tumor biomarkers M (Q1, Q3) | ||||
| CEA/(ng·mL-1) | 4.39 (2.07, 13.33) | 3.96 (2.08, 11.38) | 0.628 | |
| CA 19-9/(U·mL-1) | 10.71 (7.58, 19.50) | 12.60 (6.83, 23.25) | 0.928 | |
| Number of recurrence n | 0.351 | |||
| At 1 year | 75 | 17 | 92 | |
| At 2 years | 82 | 17 | 99 | |
| At 3 years | 85 | 17 | 102 | |
| Follow-time/month M (95% CI) | 25 (22.49, 27.51) | 19 (18.43, 19.57) | ||
| Pre-RFA characteristics of lung metastases | ||||
| Nodule size/mm n | 0.064 | |||
| <10 | 156 | 35 | 191 | |
| 10-19 | 118 | 31 | 149 | |
| 20-30 | 49 | 12 | 61 | |
| Location n | 0.183 | |||
| RUL | 76 | 15 | 91 | |
| RML | 36 | 8 | 44 | |
| RLL | 63 | 10 | 73 | |
| LUL | 77 | 18 | 95 | |
| LLL | 71 | 27 | 98 | |
| Distance 1/cm n | 0.454 | |||
| ≥1 | 266 | 67 | 333 | |
| <1 | 57 | 11 | 68 | |
| Distance 2/cm n | 0.399 | |||
| ≥1 | 124 | 34 | 158 | |
| <1 | 199 | 44 | 243 | |
| Immediate post-RFA characteristics | ||||
| Immediate pneumothorax n | 0.851 | |||
| Yes | 82 | 19 | 101 | |
| No | 241 | 59 | 300 | |
| Intra-alveolar hemorrhage n | 0.778 | |||
| Yes | 86 | 22 | 108 | |
| No | 237 | 56 | 293 | |
| Electrode chosen for RFA n | 0.722 | |||
| Expandable | 303 | 74 | 377 | |
| Straight | 20 | 4 | 24 |
Tab. 2
Univariate and multivariate COX regression analysis of clinical and radiogical features"
| Characteristic | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Clinical features | |||||
| Gender | |||||
| Male | 1.055 (0.687-1.620) | 0.806 | |||
| Female | 1 | ||||
| Age | 1.003 (0.983-1.023) | 0.748 | |||
| Initial tumor location | |||||
| Rectum | 1 | ||||
| Sigmoid-left colon | 0.663 (0.318-1.383) | 0.273 | |||
| Transverse-right colon | 0.950 (0.532-1.695) | 0.862 | |||
| Caecum* | - | - | |||
| Lymphadenopathy at diagnosis | |||||
| Yes | 1.034 (0.659-1.621) | 0.884 | |||
| No | 1 | ||||
| Systemic therapy | |||||
| Yes | 0.817 (0.524-1.275) | 0.374 | |||
| No | 1 | ||||
| Tumor biomarkers | |||||
| CEA/(ng·mL-1) | 1.004 (1.001-1.007) | 0.002 | 1.052 (1.021-1.084) | 0.001 | |
| CA 19-9/(U·mL-1) | 1.003 (1.000-1.007) | 0.062 | 1.047 (1.008-1.088) | 0.019 | |
| Pre-RFA features of the lung metastases | |||||
| Location | |||||
| RUL | 1.035 (0.506-2.112) | 0.925 | 1.327 (0.660-2.668) | 0.427 | |
| RML | 1.612 (0.723-3.594) | 0.243 | 1.645 (0.760-3.564) | 0.207 | |
| RLL | 2.588 (1.358-4.929) | 0.004 | 3.055 (1.629-5.732) | 0.001 | |
| LUL | 1 | 1 | |||
| LLL | 1.732 (0.892-3.363) | 0.104 | 2.236 (1.192-4.193) | 0.012 | |
| Distance 1/cm | |||||
| ≥1 | 1 | ||||
| <1 | 1.326 (0.789-2.230) | 0.287 | |||
| Distance 2/cm | |||||
| ≥1 | 1 | ||||
| <1 | 1.206 (0.774-1.879) | 0.409 | |||
| Immediate post-RFA features | |||||
| Pneumothorax | |||||
| Yes | 1.207 (0.758-1.923) | 0.428 | |||
| No | 1 | ||||
| Hemorrhage | |||||
| Yes | 0.812 (0.492-1.339) | 0.414 | |||
| No | 1 | ||||
| Electrode | |||||
| Expandable | 0.480 (0.240-0.958) | 0.037 | 2.171 (1.176-4.005) | 0.013 | |
| Straight | 1 | 1 | |||
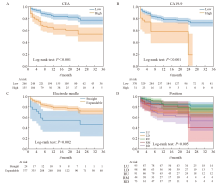
Fig. 4
Kaplan-Meier curves according to independent risk factors A: The risk stratification curves of CEA (optimal cutoff value was 6.28 ng/mL); B: The risk stratification curves of CA19-9 (optimal cutoff value was 74.72 U/mL); C: The risk stratification curves of ablation probe type; D: The risk stratification curves of position of lung metastases."
Tab. 3
Radiomics features screened by MRMRA and LASSO regression"
| Pre-RFA radiomics feature | Post-RFA radiomics feature |
|---|---|
| Shape_Elongation | Shape_Sphericity |
| Shape_MeshVolume | GLCM_Idn* |
| FirstOrder_Energy* | GLCM_Idmn |
| FirstOrder_Entropy* | GLDM_SmallDependenceLowGrayLevelEmphasis* |
| FirstOrder_MeanAbsoluteDeviation* | GLSZM_HighGrayLevelZoneEmphasis |
| FirstOrder_Skewness | NGTDM_Strength |
| FirstOrder_RootMeanSquared | NGTDM_Coarseness* |
| FirstOrder_RobustMeanAbsoluteDeviation | |
| GLCM_ClusterShade* | |
| GLCM_ClusterProminence | |
| GLCM_Idmn | |
| GLCM_Imc1* | |
| GLCM_InverseVariance* | |
| GLDM_DependenceEntropy* | |
| GLDM_GrayLevelNonUniformity* | |
| GLSZM_LargeAreaEmphasis | |
| GLSZM_LargeAreaLowGrayLevelEmphasis* |

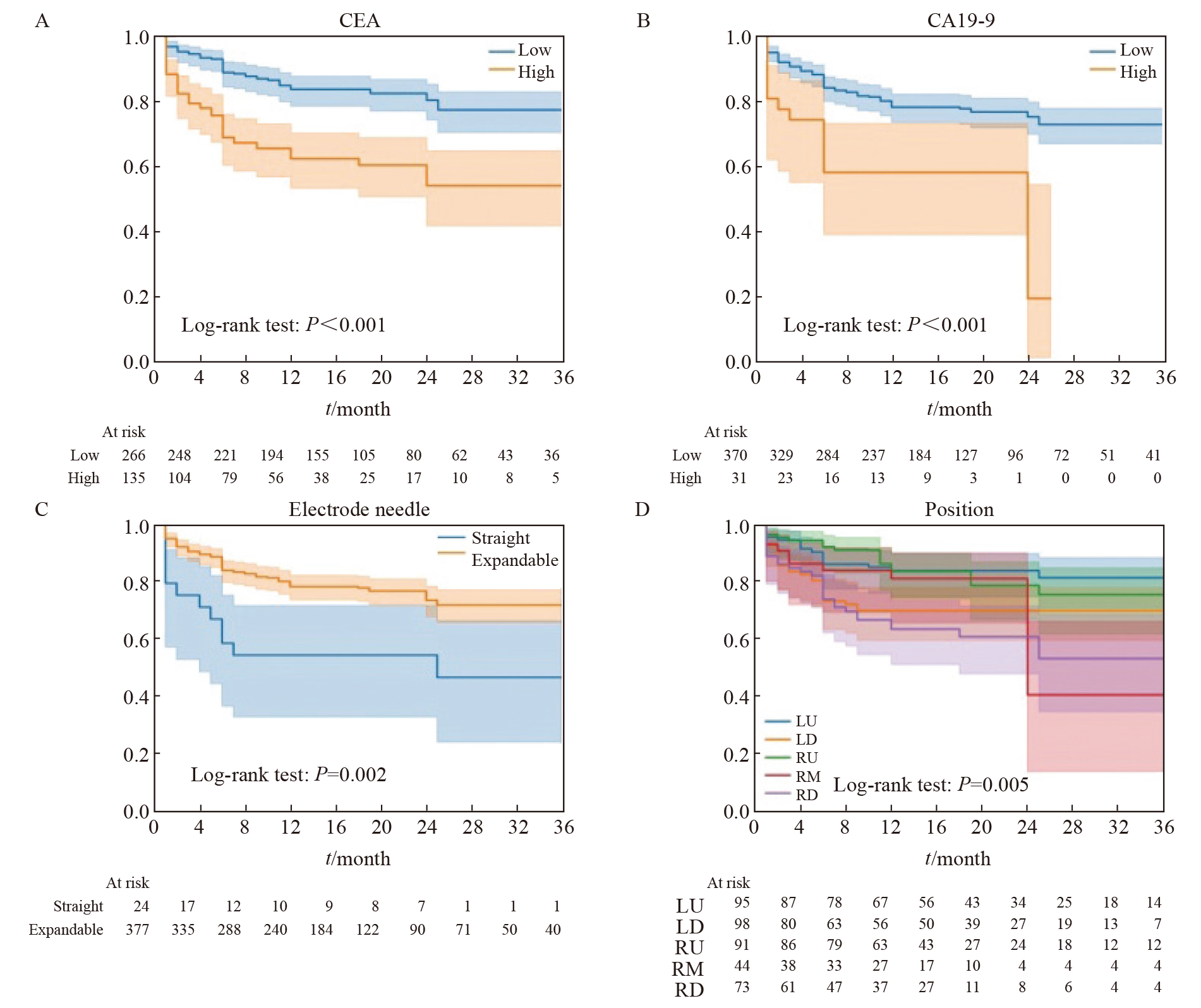
Fig. 5
Radiomics features screened by LASSO regression A: The coefficient of radiomic features changes with α. B: The C-index of the model changes with α, and the yellow line represents the α value corresponding to the highest value of the C-index in the cross-validation results. C: The correlation coefficient of radiomic features screened by LASSO regression. The prefix “before_”: The radiomic features of the preoperative CT images of RFA; The prefix “sp_”: The radiomics features of the CT images immediately after RFA."
Tab. 4
Performance comparison of each model"
| Variable | Fusion model | Radiomic model | Clinical model |
|---|---|---|---|
| Training dataset | |||
| C-index (95% CI) | 0.890 (0.854-0.927) | 0.867 (0.829-0.906) | 0.665 (0.594-0.737) |
| Brier score (1-year) | 0.092 | 0.106 | 0.161 |
| Brier score (2-year) | 0.160 | 0.189 | 0.180 |
| Brier score (3-year) | 0.161 | 0.198 | 0.177 |
| AUC (1-year) | 0.917 | 0.899 | 0.670 |
| AUC (2-year) | 0.869 | 0.850 | 0.668 |
| AUC (3-year) | 0.857 | 0.839 | 0.659 |
| Test dataset | |||
| C-index (95% CI) | 0.843 (0.768-0.916) | 0.811 (0.722-0.897) | 0.688 (0.565-0.811) |
| Brier score (1-year) | 0.126 | 0.133 | 0.172 |
| Brier score (2-year) | 0.204 | 0.241 | 0.191 |
| Brier score (3-year) | 0.174 | 0.188 | 0.187 |
| AUC (1-year) | 0.857 | 0.810 | 0.711 |
| AUC (2-year) | 0.709 | 0.618 | 0.687 |
| AUC (3-year) | 0.757 | 0.674 | 0.655 |
| [1] | SUNG H, FERLAY J, SIEGEL R L, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2021, 71(3): 209-249. |
| [2] | NORDHOLM-CARSTENSEN A, KRARUP P M, JORGENSEN L N, et al. Occurrence and survival of synchronous pulmonary metastases in colorectal cancer: a nationwide cohort study[J]. Eur J Cancer, 2014, 50(2): 447-456. |
| [3] | SIEGEL R L, MILLER K D, JEMAL A. Cancer statistics, 2019[J]. CA, 2019, 69(1): 7-34. |
| [4] |
CHO J H, KIM S, NAMGUNG M, et al. The prognostic importance of the number of metastases in pulmonary metastasectomy of colorectal cancer[J]. World J Surg Oncol, 2015, 13: 222.
doi: 10.1186/s12957-015-0621-7 pmid: 26205014 |
| [5] |
IBRAHIM T, TSELIKAS L, YAZBECK C, et al. Systemic versus local therapies for colorectal cancer pulmonary metastasis: what to choose and when?[J]. J Gastrointest Cancer, 2016, 47(3): 223-231.
doi: 10.1007/s12029-016-9818-4 pmid: 27080402 |
| [6] |
QI H, FAN W J. Value of ablation therapy in the treatment of lung metastases[J]. Thorac Cancer, 2018, 9(2): 199-207.
doi: 10.1111/1759-7714.12567 pmid: 29193688 |
| [7] |
YU W S, BAE M K, CHOI J K, et al. Pulmonary metastasectomy in colorectal cancer: a population-based retrospective cohort study using the Korean national health insurance database[J]. Cancer Res Treat, 2021, 53(4): 1104-1112.
doi: 10.4143/crt.2020.1213 pmid: 33494126 |
| [8] |
NAJAFI A, BAERE T D, PURENNE E, et al. Risk factors for local tumor progression after RFA of pulmonary metastases: a matched case-control study[J]. Eur Radiol, 2021, 31(7): 5361-5369.
doi: 10.1007/s00330-020-07675-y pmid: 33474569 |
| [9] | CERVANTES A, ADAM R, ROSELLÓ S, et al. Metastatic colorectal cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up[J]. Ann Oncol, 2023, 34(1): 10-32. |
| [10] |
YANG Q X, QI H, ZHANG R, et al. Risk factors for local progression after percutaneous radiofrequency ablation of lung tumors: evaluation based on a review of 147 tumors[J]. J Vasc Interv Radiol, 2017, 28(4): 481-489.
doi: S1051-0443(16)30842-9 pmid: 28111196 |
| [11] | DOO F X, VOSSHENRICH J, COOK T S, et al. Environmental sustainability and AI in radiology: a double-edged sword[J]. Radiology, 2024, 310(2): e232030. |
| [12] |
BRANCATO V, ESPOSITO G, COPPOLA L, et al. Standardizing digital biobanks: Integrating imaging, genomic, and clinical data for precision medicine[J]. J Transl Med, 2024, 22(1): 136.
doi: 10.1186/s12967-024-04891-8 pmid: 38317237 |
| [13] | TERRANOVA N, VENKATAKRISHNAN K. Machine learning in modeling disease trajectory and treatment outcomes: an emerging enabler for model-informed precision medicine[J]. Clin Pharmacol Ther, 2024, 115(4): 720-726. |
| [14] |
BEIG N, KHORRAMI M, ALILOU M, et al. Perinodular and intranodular radiomic features on lung CT images distinguish adenocarcinomas from granulomas[J]. Radiology, 2019, 290(3): 783-792.
doi: 10.1148/radiol.2018180910 pmid: 30561278 |
| [15] |
ABTIN F G, ERADAT J, GUTIERREZ A J, et al. Radiofrequency ablation of lung tumors: imaging features of the postablation zone[J]. Radiographics, 2012, 32(4): 947-969.
doi: 10.1148/rg.324105181 pmid: 22786987 |
| [16] | 刘宝东, 支修益. 影像引导下热消融治疗肺部肿瘤的局部疗效评价[J]. 中国医学前沿杂志(电子版), 2015, 7(2): 11-14. |
| [17] | LIU B D, ZHI X Y. Evaluation of local curative effect of image-guided thermal ablation for lung tumors[J]. Chin J Front Med Sci Electron Version, 2015, 7(2): 11-14. |
| [18] | STARMANS M P A, VAN DER VOORT S R, PHIL T, et al. Reproducible radiomics through automated machine learning validated on twelve clinical applications[EB/OL]. (2022-07-29) [2024-05-07]. http://arxiv.org/abs/2108.08618 |
| [19] |
RIGATTI S J. Random forest[J]. J Insur Med, 2017, 47(1): 31-39.
doi: 10.17849/insm-47-01-31-39.1 pmid: 28836909 |
| [20] | QIAN L Q, ZHOU Y J, ZENG W Q, et al. A random forest algorithm predicting model combining intraoperative frozen section analysis and clinical features guides surgical strategy for peripheral solitary pulmonary nodules[J]. Transl Lung Cancer Res, 2022, 11(6): 1132-1144. |
| [21] | KHUSHI M, SHAUKAT K, ALAM T M, et al. A comparative performance analysis of data resampling methods on imbalance medical data[J]. IEEE Access, 2021, 9: 109960-109975. |
| [22] | HALL D L, LLINAS J. An introduction to multisensor data fusion[J]. Proc IEEE, 1997, 85(1): 6-23. |
| [23] | RASHINKAR P, KRUSHNASAMY V S. An overview of data fusion techniques[C]. Bengaluru: ICIMIA, 2017: 694-697. |
| [24] |
MARKICH R, PALUSSIÈRE J, CATENA V, et al. Radiomics complements clinical, radiological, and technical features to assess local control of colorectal cancer lung metastases treated with radiofrequency ablation[J]. Eur Radiol, 2021, 31(11): 8302-8314.
doi: 10.1007/s00330-021-07998-4 pmid: 33954806 |
| [25] |
BAÈRE T D, AUPÉRIN A, DESCHAMPS F, et al. Radiofrequency ablation is a valid treatment option for lung metastases: experience in 566 patients with 1037 metastases[J]. Ann Oncol, 2015, 26(5): 987-991.
doi: S0923-7534(19)31500-5 pmid: 25688058 |
| [26] |
GONZALEZ M, PONCET A, COMBESCURE C, et al. Risk factors for survival after lung metastasectomy in colorectal cancer patients: a systematic review and meta-analysis[J]. Ann Surg Oncol, 2013, 20(2): 572-579.
doi: 10.1245/s10434-012-2726-3 pmid: 23104709 |
| [27] |
PALUSSI RE J, MARCET B, DESCAT E, et al. Lung tumors treated with percutaneous radiofrequency ablation: computed tomography imaging follow-up[J]. Cardiovasc Intervent Radiol, 2011, 34(5): 989-997.
doi: 10.1007/s00270-010-0048-z pmid: 21127867 |
| [28] | MAPELLI P, BEZZI C, MUFFATTI F, et al. Preoperative assessment of lymph nodal metastases with[68Ga]Ga-DOTATOC PET radiomics for improved surgical planning in well-differentiated pancreatic neuroendocrine tumours[J]. Eur J Nucl Med Mol Imaging, 2024, 51(9): 2774-2783. |
| [29] |
ZHU F D, YANG C, XIA Y, et al. CT-based radiomics models may predict the early efficacy of microwave ablation in malignant lung tumors[J]. Cancer Imaging, 2023, 23(1): 60.
doi: 10.1186/s40644-023-00571-w pmid: 37308918 |
| [30] |
HUANG H Z, CHEN H, ZHENG D Z, et al. Habitat-based radiomics analysis for evaluating immediate response in colorectal cancer lung metastases treated by radiofrequency ablation[J]. Cancer Imaging, 2024, 24(1): 44.
doi: 10.1186/s40644-024-00692-w pmid: 38532520 |
| [31] | BENSON A B, VENOOK A P, AL-HAWARY M M, et al. Colon cancer, version 2.2021, NCCN clinical practice guidelines in oncology[J]. J Natl Compr Canc Netw, 2021, 19(3): 329-359. |
| [32] |
DUFFY M J, LAMERZ R, HAGLUND C, et al. Tumor markers in colorectal cancer, gastric cancer and gastrointestinal stromal cancers: European group on tumor markers 2014 guidelines update[J]. Int J Cancer, 2014, 134(11): 2513-2522.
doi: 10.1002/ijc.28384 pmid: 23852704 |
| [33] | LIU B, LI C H, SUN X R, et al. Assessment and prognostic value of immediate changes in post-ablation intratumor density heterogeneity of pulmonary tumors via radiomics-based computed tomography features[J]. Front Oncol, 2021, 11: 615174. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
沪ICP备12009617
Powered by Beijing Magtech Co. Ltd
