Welcome to China Oncology,
China Oncology ›› 2025, Vol. 35 ›› Issue (3): 309-319.doi: 10.19401/j.cnki.1007-3639.2025.03.007
• Article • Previous Articles Next Articles
ZHENG Wentian( ), GONG Hui, ZHANG Xinyue, HAO Jiayi, WANG Yajie, JIANG Yingying(
), GONG Hui, ZHANG Xinyue, HAO Jiayi, WANG Yajie, JIANG Yingying( )
)
Received:2024-07-30
Revised:2025-02-06
Online:2025-03-30
Published:2025-04-10
Contact:
JIANG Yingying
Supported by:Share article
CLC Number:
ZHENG Wentian, GONG Hui, ZHANG Xinyue, HAO Jiayi, WANG Yajie, JIANG Yingying. Effects of SEC14L1P1 on proliferation and migration of oral squamous cell carcinoma cells[J]. China Oncology, 2025, 35(3): 309-319.
Tab. 1
The sequences of the primers used in RTFQ-PCR"
| Gene | Primer sequence |
|---|---|
| SEC14L1P1 | Forward: 5‘-GGCCTGCTGGATTACATTGATAG-3‘ |
| Reverse: 5‘-CCAGTAGAGAGATTTGGGGACC-3‘ | |
| β-actin | Forward: 5‘-CATGTACGTTGCTATCCAGGC-3‘ |
| Reverse: 5‘-CTCCTTAATGTCACGCACGAT-3‘ | |
| GAPDH | Forward: 5‘-GAACGGGAAGCTCACTGG-3‘ |
| Reverse: 5‘-GCCTGCTTCACCACCTTCT-3‘ | |
| U6 | Forward: 5‘-CTCGCTTCGGCAGCACATATACT-3‘ |
| Reverse: 5‘-ATTTGCGTGTCATCCTTGCGCA-3‘ | |
| E-cadherin | Forward: 5‘-CGAGAGCTACACGTTCACGG-3‘ |
| Reverse: 5‘-GGGTGTCGAGGGAAAAATAGG-3‘ | |
| N-cadherin | Forward: 5‘-TGCGGTACAGTGTAACTGGG-3‘ |
| Reverse: 5‘-GAAACCGGGCTATCTGCTCG-3‘ | |
| Vimentin | Forward: 5‘-AGTCCACTGAGTACCGGAGAC-3‘ |
| Reverse: 5‘-CATTTCACGCATCTGGCGTTC-3‘ |

Fig. 1
Expression and prognosis of SEC14L1P1 in HNSCC tissue A: ENCORI database analysis of SEC14L1P1 expression in HNSCC; B, C: GDC database analysis of SEC14L1P1 expression in HNSCC tissues and normal tissues; D: ROC curves analysis of the diagnostic value of SEC14L1P1 in HNSCC; E, F: UCSC Xena database analysis of SEC14L1P1 expression and patient survival rate in HNSCC. ****: P<0.000 1."
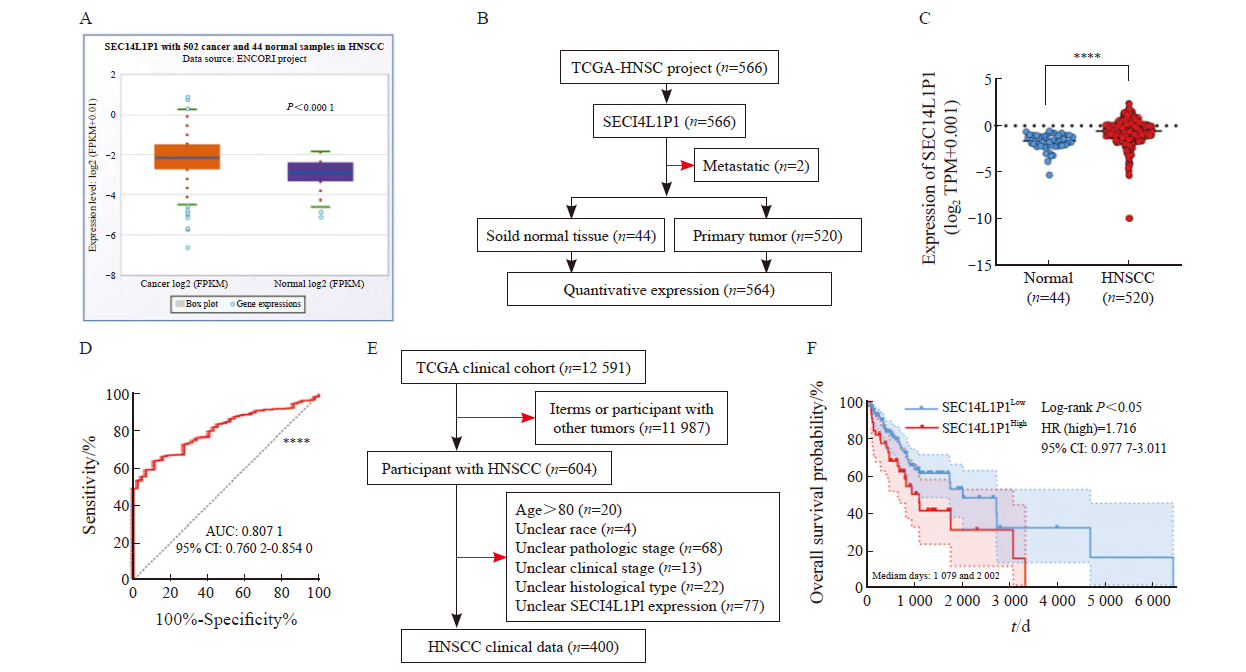

Fig. 3
Effect of SEC14L1P1 knockdown on proliferation and migration of CAL-27 cells A: RTFQ-PCR to detect the relative expression of SEC14L1P1 knockdown; B: CCK-8 assay to detect the effect of SEC14L1P1 knockdown on the proliferative ability of CAL-27 cells; C, D: Transwell migration assay to detect the cell migration ability of SEC14L1P1 knockdown. **: P<0.01; ***: P <0.001; ****: P<0.000 1."
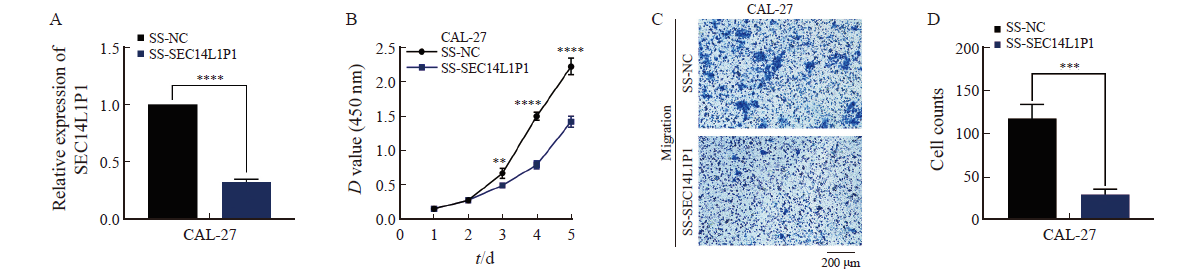

Fig. 4
Effect of overexpression of SEC14L1P1 on proliferation and migration of HN30 cells A: RTFQ-PCR to detect the relative expression of SEC14L1P1 after its overexpression; B: CCK-8 assay to detect the effect of SEC14L1P1 overexpression on the proliferative ability of HN30 cells; C, D: Transwell migration assay to detect the migration ability of cells after the overexpression of SEC14L1P1. **: P<0.01; ***: P<0.001; ****: P <0.000 1."
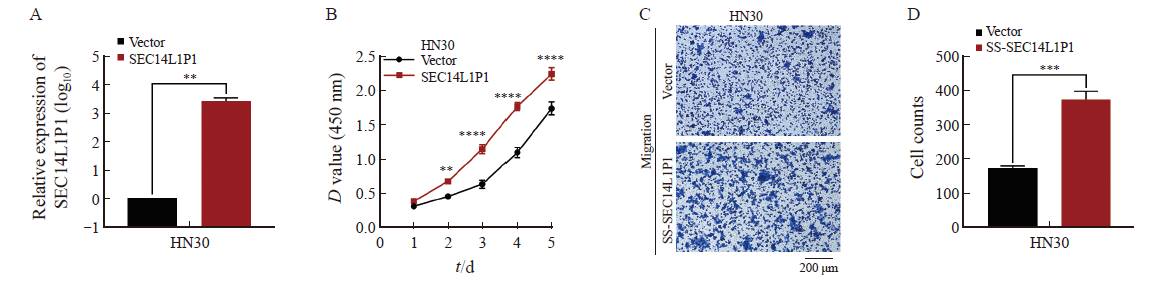

Fig. 5
Effects of SEC14L1P1 knockdown/overexpression on EMT in OSCC cells A, B: RTFQ-PCR to detect the effect of SEC14L1P1 knockdown/overexpression on EMT-related proteins; C, D, E: Western blot to detect the effect of SEC14L1P1 knockdown/overexpression on EMT-related protein expression. *: P<0.05; **: P<0.01; ****: P<0.000 1."

Fig. 6
Effect of SEC14L1P1 knockdown on the growth of subcutaneous xenograft tumors formed by OSCC cells in nude mice A: Effect of SEC14L1P1 knockdown on CAL-27 cell tumor size; B: Effect of SEC14L1P1 knockdown on tumor weight in CAL-27 cells; C: H-E staining and Ki-67 immunohistochemical staining of CAL-27 cell xenograft tumor tissues after SEC14L1P1 knockdown. ***: P<0.001."
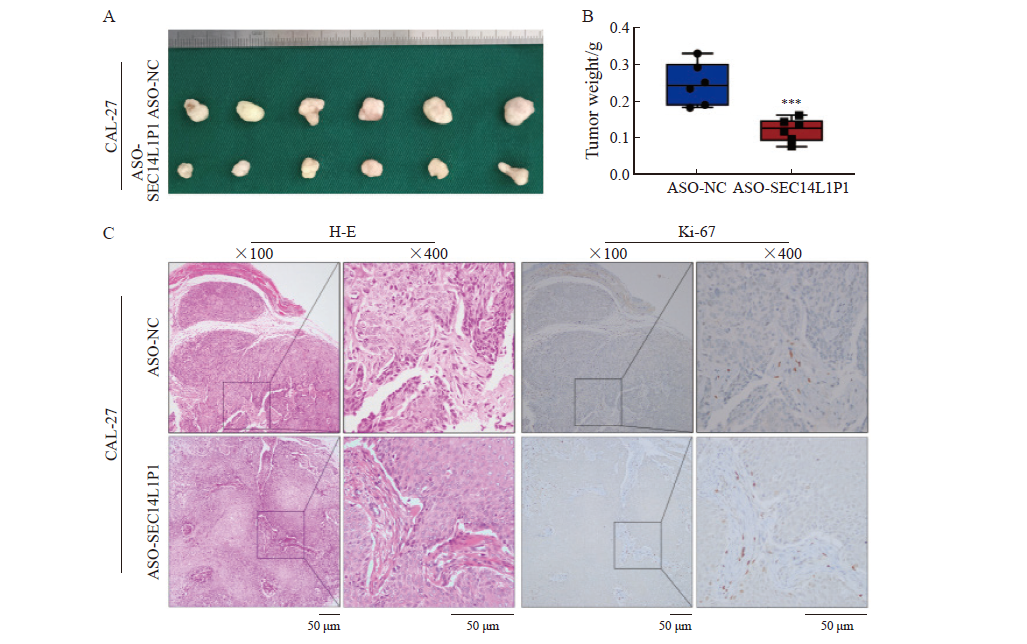

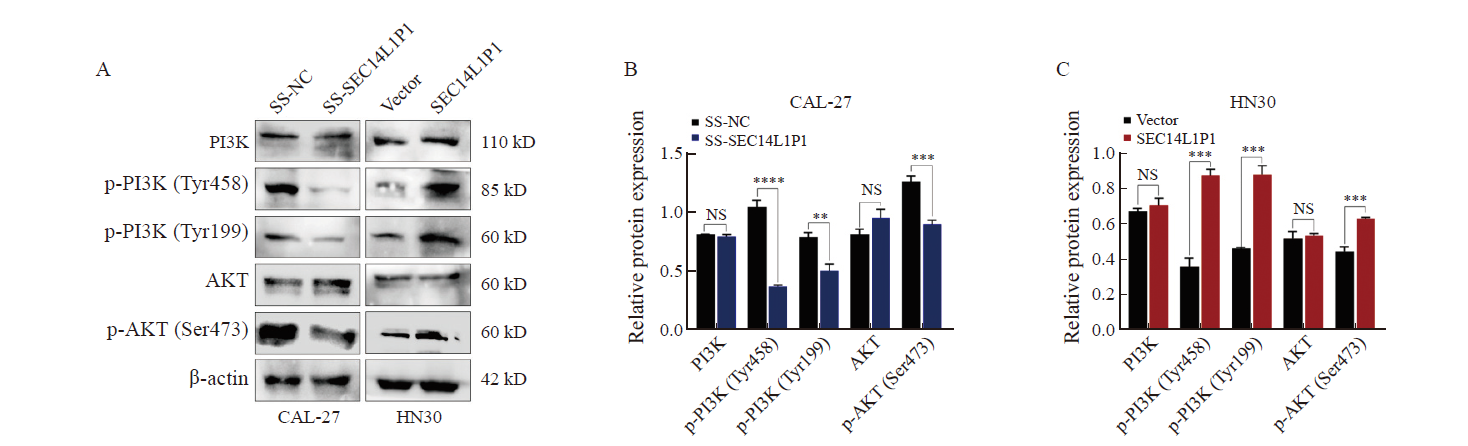
Fig. 7
Mechanistic study of SEC14L1P1 regulation of the PI3K/AKT signaling pathway A, B, C: Western blot to detect the effect of SEC14L1P1 knockdown/overexpression on the expression of PI3K/AKT and p-PI3K/p-AKT protein expression. **: P<0.01; ***: P<0.001; ****: P<0.000 1; NS: No significance."
| [1] |
JING F Y, ZHU L J, ZHANG J Y, et al. Multi-omics reveals lactylation-driven regulatory mechanisms promoting tumor progression in oral squamous cell carcinoma[J]. Genome Biol, 2024, 25(1): 272.
doi: 10.1186/s13059-024-03383-8 pmid: 39407253 |
| [2] | WANG D W, QI H, ZHANG H X, et al. TAF1L promotes development of oral squamous cell carcinoma via decreasing autophagy-dependent apoptosis[J]. Int J Biol Sci, 2020, 16(7): 1180-1193. |
| [3] |
HAO Y L, XIAO Y X, LIAO X Y, et al. FGF8 induces epithelial-mesenchymal transition and promotes metastasis in oral squamous cell carcinoma[J]. Int J Oral Sci, 2021, 13(1): 6.
doi: 10.1038/s41368-021-00111-x pmid: 33649301 |
| [4] | BRIDGES M C, DAULAGALA A C, KOURTIDIS A. LNCcation: lncRNA localization and function[J]. J Cell Biol, 2021, 220(2): e202009045. |
| [5] | JIANG Y Y, GUO H Y, TONG T, et al. lncRNA lnc-POP1-1 upregulated by VN1R5 promotes cisplatin resistance in head and neck squamous cell carcinoma through interaction with MCM5[J]. Mol Ther, 2022, 30(1): 448-467. |
| [6] | CHEN Y, LU Y W, YANG L, et al. LncRNA like NMRK2 mRNA functions as a key molecular scaffold to enhance mitochondrial respiration of NONO-TFE3 rearranged renal cell carcinoma in an NAD+ kinase-independent manner[J]. J Exp Clin Cancer Res, 2023, 42(1): 252. |
| [7] | XIE J B, LI Y, LIU X M, et al. Evolutionary origins of pseudogenes and their association with regulatory sequences in plants[J]. Plant Cell, 2019, 31(3): 563-578. |
| [8] | LU N, JIANG Q M, XU T S, et al. LncOCMRL1 promotes oral squamous cell carcinoma growth and metastasis via the RRM2/EMT pathway[J]. J Exp Clin Cancer Res, 2024, 43(1): 267. |
| [9] | 蒋英英, 陈曦, 石雨, 等. 长链非编码RNA COL11A1-208对口腔鳞癌细胞增殖及侵袭的影响[J]. 解放军医学杂志, 2022, 47(9): 851-862. |
| JIANG Y Y, CHEN X, SHI Y, et al. Effect of long non-coding RNA COL11A1-208 on proliferation and invasion in oral squamous cell carcinoma cells[J]. Med J Chin People’s Liberation Army, 2022, 47(9): 851-862. | |
| [10] |
ZHOU J Y, HU Z X, WANG L, et al. Tumor-colonized Streptococcus mutans metabolically reprograms tumor microenvironment and promotes oral squamous cell carcinoma[J]. Microbiome, 2024, 12(1): 193.
doi: 10.1186/s40168-024-01907-9 pmid: 39369210 |
| [11] | ZHOU W K, FENG Y S, LIN C Z, et al. Yin Yang 1-induced long noncoding RNA DUXAP9 drives the progression of oral squamous cell carcinoma by blocking CDK1-mediated EZH2 degradation[J]. Adv Sci (Weinh), 2023, 10(25): e2207549. |
| [12] |
JIANG Y Y, CAO W, WU K, et al. LncRNA LINC00460 promotes EMT in head and neck squamous cell carcinoma by facilitating peroxiredoxin-1 into the nucleus[J]. J Exp Clin Cancer Res, 2019, 38(1): 365.
doi: 10.1186/s13046-019-1364-z pmid: 31429766 |
| [13] | JIANG Y Y, WU K, CAO W, et al. Long noncoding RNA KTN1-AS1 promotes head and neck squamous cell carcinoma cell epithelial-mesenchymal transition by targeting miR-153-3p[J]. Epigenomics, 2020, 12(6): 487-505. |
| [14] | YAO H Y, LU Y Q, YANG X L, et al. Arabidopsis Sec14 proteins (SFH5 and SFH7) mediate interorganelle transport of phosphatidic acid and regulate chloroplast development[J]. Proc Natl Acad Sci USA, 2023, 120(6): e2221637120. |
| [15] | JIANG Z M, YANG G C, WANG G C, et al. SEC14L3 knockdown inhibited clear cell renal cell carcinoma proliferation, metastasis and sunitinib resistance through an SEC14L3/RPS3/NFκB positive feedback loop[J]. J Exp Clin Cancer Res, 2024, 43(1): 288. |
| [16] | DE SANCTIS P, FILARDO G, ABRUZZO P M, et al. Non-coding RNAs in the transcriptional network that differentiates skeletal muscles of sedentary from long-term endurance- and resistance-trained elderly[J]. Int J Mol Sci, 2021, 22(4): 1539. |
| [17] | CRISTIANO L. The pseudogenes of eukaryotic translation elongation factors (EEFs): role in cancer and other human diseases[J]. Genes Dis, 2021, 9(4): 941-958. |
| [18] | SUN M Y, CHANG L, HE L, et al. Combining single-cell profiling and functional analysis explores the role of pseudogenes in human early embryonic development[J]. J Genet Genomics, 2024, 51(11): 1173-1186. |
| [19] | QU W B, ZHOU X, JIANG X J, et al. Long noncoding RNA Gpr137b-ps promotes advanced atherosclerosis via the regulation of autophagy in macrophages[J]. Arterioscler Thromb Vasc Biol, 2023, 43(11): e468-e489. |
| [20] | WU T, GU W Z, HONG L B, et al. Exploration of shared TF-miRNA-mRNA and mRNA-RBP-pseudogene networks in type 2 diabetes mellitus and breast cancer[J]. Front Immunol, 2022, 13: 915017. |
| [21] | MA Y N, LIU S Q, GAO J, et al. Genome-wide analysis of pseudogenes reveals HBBP1’s human-specific essentiality in erythropoiesis and implication in β-thalassemia[J]. Dev Cell, 2021, 56(4): 478-493.e11. |
| [22] | GUSTAVSSON E K, SETHI S, GAO Y J, et al. The annotation of GBA1 has been concealed by its protein-coding pseudogene GBAP1[J]. Sci Adv, 2024, 10(26): eadk1296. |
| [23] | HUANG H W, CHEN C Y, HUANG Y H, et al. CMAHP promotes metastasis by reducing ubiquitination of snail and inducing angiogenesis via GM-CSF overexpression in gastric cancer[J]. Oncogene, 2022, 41(2): 159-172. |
| [24] | ZHENG L F, GUO Q Q, XIANG C X, et al. Transcriptional factor six2 promotes the competitive endogenous RNA network between CYP4Z1 and pseudogene CYP4Z2P responsible for maintaining the stemness of breast cancer cells[J]. J Hematol Oncol, 2019, 12(1): 23. |
| [25] | GAO X, QIN T, MAO J, et al. PTENP1/miR-20a/PTEN axis contributes to breast cancer progression by regulating PTEN via PI3K/AKT pathway[J]. J Exp Clin Cancer Res, 2019, 38(1): 256. |
| [26] | SUN C C, ZHANG L, LI G, et al. The lncRNA PDIA3P interacts with miR-185-5p to modulate oral squamous cell carcinoma progression by targeting cyclin D2[J]. Mol Ther Nucleic Acids, 2017, 9: 100-110. |
| [27] |
DING T Y, XU H W, ZHANG X Y, et al. Prohibitin 2 orchestrates long noncoding RNA and gene transcription to accelerate tumorigenesis[J]. Nat Commun, 2024, 15(1): 8385.
doi: 10.1038/s41467-024-52425-z pmid: 39333493 |
| [28] | YIN Y F, LU J Y, ZHANG X C, et al. U1 snRNP regulates chromatin retention of noncoding RNAs[J]. Nature, 2020, 580(7801): 147-150. |
| [29] |
CASTANEDA M, DEN HOLLANDER P, KUBURICH N A, et al. Mechanisms of cancer metastasis[J]. Semin Cancer Biol, 2022, 87: 17-31.
doi: 10.1016/j.semcancer.2022.10.006 pmid: 36354098 |
| [30] | HUANG Y H, HONG W Q, WEI X W. The molecular mechanisms and therapeutic strategies of EMT in tumor progression and metastasis[J]. J Hematol Oncol, 2022, 15(1): 129. |
| [31] |
JOHN LIU S, DANG H X, LIM D A, et al. Long noncoding RNAs in cancer metastasis[J]. Nat Rev Cancer, 2021, 21(7): 446-460.
doi: 10.1038/s41568-021-00353-1 pmid: 33953369 |
| [32] |
GLAVIANO A, FOO A S C, LAM H Y, et al. PI3K/AKT/mTOR signaling transduction pathway and targeted therapies in cancer[J]. Mol Cancer, 2023, 22(1): 138.
doi: 10.1186/s12943-023-01827-6 pmid: 37596643 |
| [33] | HE Y, SUN M M, ZHANG G G, et al. Targeting PI3K/Akt signal transduction for cancer therapy[J]. Signal Transduct Target Ther, 2021, 6(1): 425. |
| [34] | MURAYAMA T, NAKAYAMA J, JIANG X P, et al. Targeting DHX9 triggers tumor-intrinsic interferon response and replication stress in small cell lung cancer[J]. Cancer Discov, 2024, 14(3): 468-491. |
| [35] | SUN Y, ZHANG H, MA R R, et al. ETS-1-activated LINC01016 over-expression promotes tumor progression via suppression of RFFL-mediated DHX9 ubiquitination degradation in breast cancers[J]. Cell Death Dis, 2023, 14(8): 507. |
| [36] |
CHI M, LIU J, MEI C X, et al. TEAD4 functions as a prognostic biomarker and triggers EMT via PI3K/AKT pathway in bladder cancer[J]. J Exp Clin Cancer Res, 2022, 41(1): 175.
doi: 10.1186/s13046-022-02377-3 pmid: 35581606 |
| [37] | LI D D, XIA L Y, HUANG P, et al. Cancer-associated fibroblast-secreted IGFBP7 promotes gastric cancer by enhancing tumor associated macrophage infiltration via FGF2/FGFR1/PI3K/AKT axis[J]. Cell Death Discov, 2023, 9(1): 17. |
| [38] | ZHAN K, PAN H F, ZHOU Z, et al. Biological role of long non-coding RNA KCNQ1OT1 in cancer progression[J]. Biomed Pharmacother, 2023, 169: 115876. |
| [39] | HUA H, ZHANG H Y, CHEN J Z, et al. Targeting Akt in cancer for precision therapy[J]. J Hematol Oncol, 2021, 14(1): 128. |
| [40] | LI Q, LI B, LU C L, et al. LncRNA LINC01857 promotes cell growth and diminishes apoptosis via PI3K/mTOR pathway and EMT process by regulating miR-141-3p/MAP4K4 axis in diffuse large B-cell lymphoma[J]. Cancer Gene Ther, 2021, 28(9): 1046-1057. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
沪ICP备12009617
Powered by Beijing Magtech Co. Ltd
