Welcome to China Oncology,
China Oncology ›› 2024, Vol. 34 ›› Issue (8): 763-776.doi: 10.19401/j.cnki.1007-3639.2024.08.006
• Review • Previous Articles Next Articles
XIAO Yi1,2( ), WU Ming1,3, YAO Gang1(
), WU Ming1,3, YAO Gang1( )
)
Received:2024-05-13
Revised:2024-07-29
Online:2024-08-30
Published:2024-09-10
Share article
CLC Number:
XIAO Yi, WU Ming, YAO Gang. Research progress and future perspectives of tumor organoid[J]. China Oncology, 2024, 34(8): 763-776.

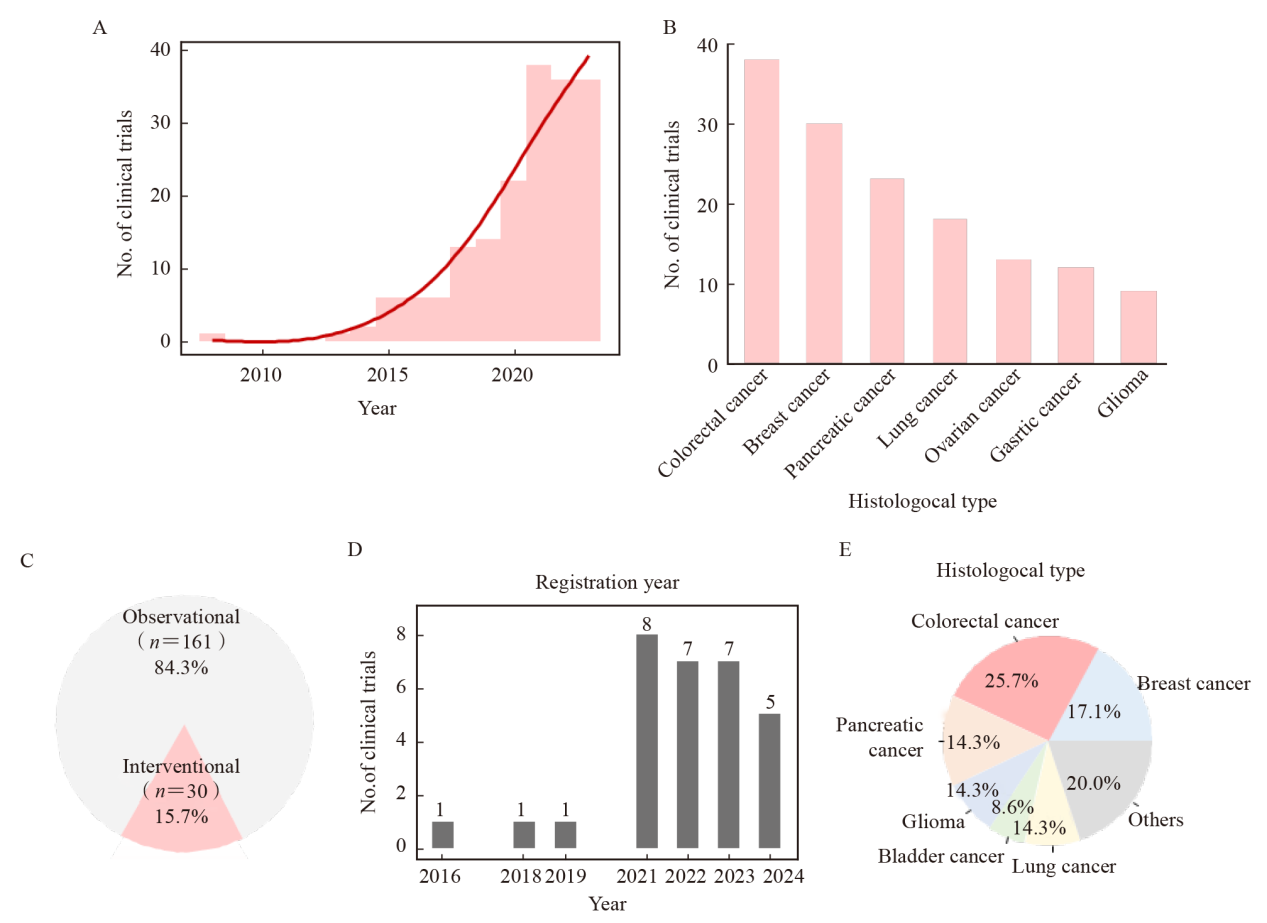
Fig. 2
Trends of clinical trials including organoids A: The number of trials including organoids registered in ClinicalTrials.gov and EU Clinical Trials Register over recent years; B: The number of trials including organoids with different histological types. Only the top seven items were illustrated; C: Proportions of the observational and interventional trials including organoids. D-E: The illustration of the registration years (D) and histological types (E) of the interventional trials."
Tab. 1
Summary of organoid-guided interventional trials"
| Identifiers | Histology | Registration year | Drug (s) | Status | Results/goals |
|---|---|---|---|---|---|
| - | Colorectal cancer[ | Unknown | A 87-drug panel | Completed | This trial enrolled 28 patients and successfully established 19 organoids, out of which 15 were utilized for drug screening. One patient underwent treatment based on the drug sensitivity results of the organoid and showed partial remission after 3 months of evaluation |
| 2014-003811-13 | Colorectal cancer[ | 2014 | Palbociclib, axitinib, selumetinib, gefitinib | Completed | This trial enrolled 54 patients and successfully established 31 organoids, of which 25 were utilized for drug screening. Out of the 25 organoids, 19 received drug sensitivity results. Based on these results, 6 patients underwent treatment; however, none of them showed any efficacy |
| NCT05842187 | Pancreatic cancer/gastric cancer | 2023 | Various clinically approved drugs | Recruiting | This trial will use organoid models to guide the precision treatment of metastatic pancreatic cancer and gastric cancer |
| NCT04931394 | Pancreatic cancer | 2021 | Gemcitabine, 5-fluorouracil, paclitaxel, oxaliplatin, irinotecan | Recruiting | This trial will use organoid models to guide the adjuvant chemotherapy of pancreatic cancer |
| NCT04931381 | Pancreatic cancer | 2021 | Gemcitabine, 5-fluorouracil, paclitaxel, oxaliplatin, irinotecan | Recruiting | This trial will use organoid models to guide the chemotherapy of advanced pancreatic cancer |
| NCT06102824 | Breast cancer | 2023 | Taxane, anthracycline, 5-fluorouracil, gemcitabine, vinorelbine, eribulin, utidelone, carboplatin, sacituzumab govitecan, and trastuzumab deruxtecan | Recruiting | This trial will use organoid models to guide the treatment of advanced breast cancer |
| NCT06268652 | Breast cancer | 2024 | Personalized drug library contains 55 drugs approved by the FDA | Recruiting | This trial will compare the efficacy of organoid-guided personalized treatment with the treatment of physician’s choice in breast cancer |
| NCT05832398 | Colorectal cancer | 2023 | Oxaliplatin, irinotecan, 5-fluorouracil | Recruiting | This trial will use organoid models to guide the precise chemotherapy of colorectal cancer |
| NCT05177432 | Breast cancer | 2021 | 10-12 anti-cancer drugs | Recruiting | This trial will use organoid models to develop a quadratic phenotypic optimization platform (QPOP) to guide the treatment of breast cancer |
| NCT05669586 | Lung cancer | 2023 | Unknown | Recruiting | This trial will use organoid models to guide the precise treatment of refractory non-small cell lung cancer |
| NCT05813509 | Ovarian cancer | 2022 | 10 potential clinical therapeutic drugs | Recruiting | This trial will use organoid models to guide the personalized treatment of ovarian cancer |
| NCT06246630 | Pancreatic neuroendocrine tumor | 2024 | Various clinically approved drugs | Not yet recruiting | This trial will use organoid models to guide the treatment of pancreatic neuroendocrine tumors |
| NCT05024734 | Bladder cancer | 2022 | Epirubicin, mitomycin, gemcitabine, docetaxel | Recruiting | This trial will use organoid models to guide the treatment of non-muscle invasive bladder cancers |
| NCT06077591 | Solid tumors | 2024 | Unknown | Not yet recruiting | This trial will use organoid models and next-generating sequencing to guide the treatment of advanced and inoperable solid tumors |
| NCT05352165 | Colorectal cancer | 2023 | Oxaliplatin, irinotecan, 5-fluorouracil | Not yet recruiting | This trial will compare the efficacy of organoid-guided neoadjuvant chemotherapy with traditional neoadjuvant chemotherapy regimens in advanced colorectal cancer |
| NCT05378048 | Abdominal tumors | 2022 | Unknown | Withdrawn | This trial will compare the efficacy of organoid-guided personalized treatment with traditional treatment strategies in advanced and inoperable abdominal tumors |
| NCT04279509 | Solid tumors | 2019 | 5-fluorouracil, carboplatin, cyclophosphamide, docetaxel, doxorubicin, gemcitabine, irinotecan, oxaliplatin, paclitaxel, vinorelbine, etoposide, ifosfamide, methotrexate, pemetrexed and topotecan | Unknown | This trial will use organoid models to conduct high-throughput drug screening to guide the chemotherapy of refractory solid tumors |
| NCT05267912 | Any cancer type | 2022 | A panel of drugs (chemotherapy, hormonal therapy, targeted therapy) | Recruiting | This trial is a multi-center study evaluating the feasibility of using organoid models to guide the precision treatment of multiple advanced tumors |
| NCT04450706 | Breast cancer | 2021 | Unknown | Recruiting | This trial will use organoid models to guide the precision treatment of metastatic breast cancers |
| NCT06057298 | Colorectal cancer | 2021 | Oxaliplatin, mitomycin | Recruiting | This trial will use organoid models to guide the hyperthermic introperitoneal chemotherapy of colorectal cancers with peritoneal metastasis |
| NCT04842006 | Colorectal cancer | 2021 | Capecitabine, oxaliplatin | Recruiting | This trial will use organoid models to guide the neoadjuvant and adjuvant therapy of colorectal cancer |
| NCT05725200 | Colorectal cancer | 2022 | Alectinib, cetuximab, crizotinib, dasatinib, everolimus, encorafenib, gemcitabine, idelalisib, larotrectinib, methotrexate, palbociclib, panobinostat, pembrolizumab, petrozumab, trastuzumab, talazoparib, venetoclax | Recruiting | This trial will use organoid models to guide the precision treatment of metastatic colorectal cancer |
| NCT05464082 | Breast cancer | 2023 | Unknown | Recruiting | This trial will use organoid models to guide the precision treatment of metastatic triple-negative breast cancers |
| NCT03778814 | Lung cancer/solid tumors | 2018 | Engineering TCR-T cells | Recruiting | This trial will use organoid models to guide the TCR-T immunotherapy of lung cancers and other solid tumors |
| NCT05429684 | Breast cancer | 2021 | Trastuzumab, pertuzumab, nab paclitaxel, pyrotinib, capecitabine, T-DM1, everolimus, CDK4/6 inhibitor, aromatase-Inhibitors, anti-PD-1 monoclonal antibody | Recruiting | This trial will use organoid models to guide the precision treatment of refractory HER2-positive breast cancers |
| NCT05381038 | Solid tumors | 2022 | Azacitidine, docetaxel, paclitaxel, irinotecan | Not yet recruiting | This trial will use organoid models to develop a QPOP and CURATE.AI, which will guide the personalized combinatory therapy with azacytidine in solid tumors. |
| NCT06227065 | Bladder cancer | 2024 | Epirubicin, mitomycin, gemcitabine, docetaxel | Not yet recruiting | This trial will use organoid models to guide the precise neoadjuvant chemotherapy of low-grade non-muscle invasive bladder cancers |
| NCT05432518 | Glioblastoma | 2023 | Afatinib, dasatinib, palbociclib, everolimus, olaparib | Recruiting | This trial will use organoid models to guide the treatment of refractory glioblastoma |
| NCT05473923 | Glioma | 2022 | Unknown | Recruiting | This trial will use organoid models to guide the precision treatment of refractory high-grade glioma |
| NCT05532397 | Astrocytoma | 2023 | Unknown | Recruiting | This trial will use organoid models to guide the combinatory therapy of refractory high-grade astrocytoma |
| 2020-003395-41 | Colorectal cancer | 2020 | Alectinib, crizotinib, dasatinib, everolimus, gemcitabine, idelalisib, larotrectinib, methotrexate, palbociclib, panobinostat, pembrolizumab, pertuzumab, trastuzumab, talazoparib, venetoclax, cetuximab, encorafinib | Recruiting | This trial will use organoid models to guide the personalized therapy of colorectal cancers |
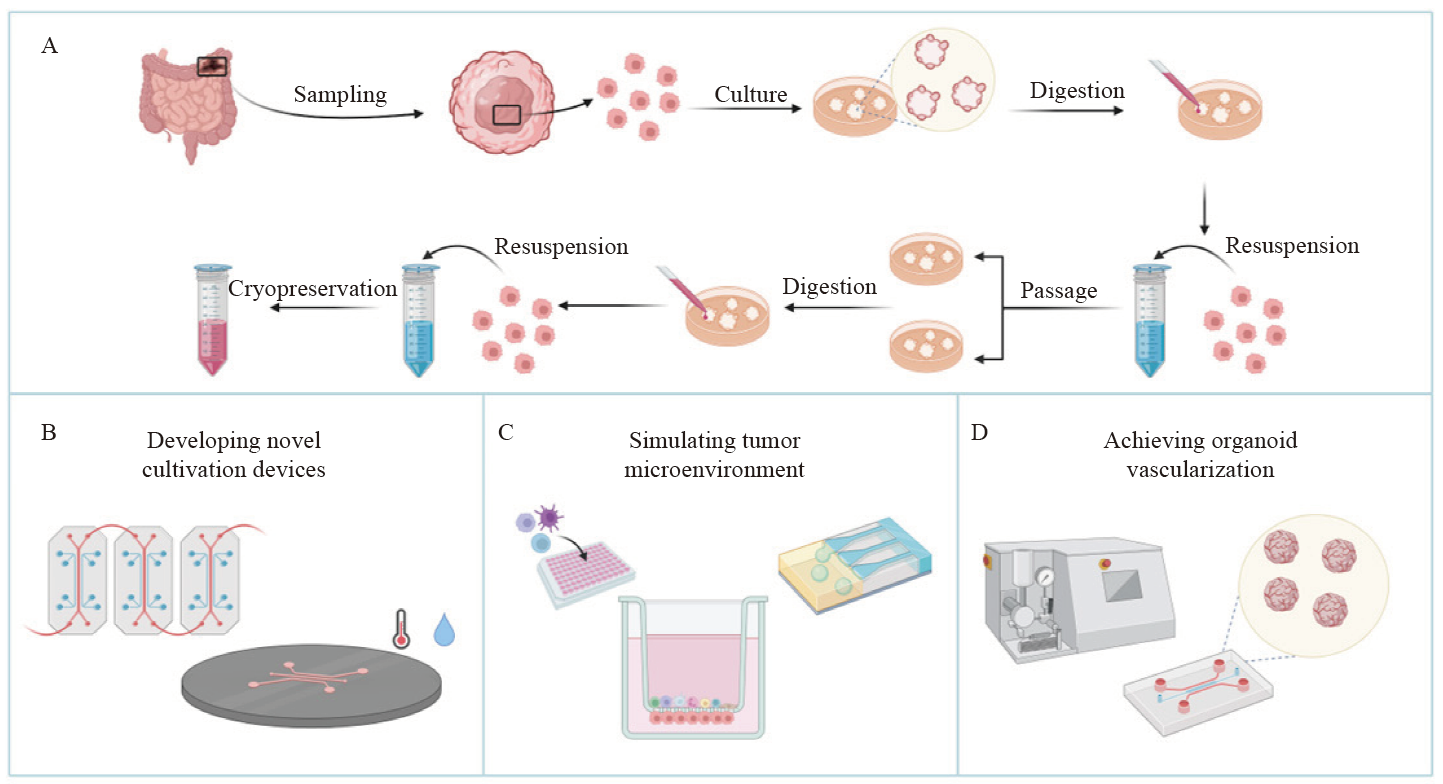
Fig. 3
Organoid culture and new technologies A: The culture process of organoid: isolation, culture, passage, and cryopreservation; B-D: New technologies in the field of organoid included microfluidics and organ-on-a-chip (B), simulating tumor microenvironment (C) and organoid vascularization (D)."
| [1] | SIEGEL R, GIAQUINTO A N, JEMAL A. Cancer statistics, 2024[J]. CA A Cancer J Clin, 2024, 74: 12-49. |
| [2] | 郑荣寿, 陈茹, 韩冰峰, 等. 2022年中国恶性肿瘤流行情况分析[J]. 中华肿瘤杂志, 2024, 46(3):221-231. |
| ZHENG R S, CHEN R, HAN B F, et al. Cancer incidence and mortality in China, 2022[J]. Chin J Oncol, 2024, 46(3): 221-231. | |
| [3] | TSIMBERIDOU A M, FOUNTZILAS E, NIKANJAM M, et al. Review of precision cancer medicine: evolution of the treatment paradigm[J]. Cancer Treat Rev, 2020, 86: 102019. |
| [4] | XU H X, JIAO D C, LIU A G, et al. Tumor organoids: applications in cancer modeling and potentials in precision medicine[J]. J Hematol Oncol, 2022, 15(1): 58. |
| [5] | FOO M A, YOU M L, CHAN S L, et al. Clinical translation of patient-derived tumour organoids-bottlenecks and strategies[J]. Biomark Res, 2022, 10(1): 10. |
| [6] |
VAN DE WETERING M, FRANCIES H E, FRANCIS J M, et al. Prospective derivation of a living organoid biobank of colorectal cancer patients[J]. Cell, 2015, 161(4): 933-945.
doi: 10.1016/j.cell.2015.03.053 pmid: 25957691 |
| [7] | PAULI C, HOPKINS B D, PRANDI D, et al. Personalized in vitro and in vivo cancer models to guide precision medicine[J]. Cancer Discov, 2017, 7(5): 462-477. |
| [8] |
WILSON H V. A new method by which sponges may be artificially reared[J]. Science, 1907, 25(649): 912-915.
pmid: 17842577 |
| [9] | CORRÒ C, NOVELLASDEMUNT L, LI V S W. A brief history of organoids[J]. Am J Physiol Cell Physiol, 2020, 319(1): C151-C165. |
| [10] | EVANS M. Origin of mouse embryonal carcinoma cells and the possibility of their direct isolation into tissue culture[J]. J Reprod Fertil, 1981, 62(2): 625-631. |
| [11] | LI M L, AGGELER J, FARSON D A, et al. Influence of a reconstituted basement membrane and its components on casein gene expression and secretion in mouse mammary epithelial cells[J]. Proc Natl Acad Sci U S A, 1987, 84(1): 136-140. |
| [12] | SATO T, VRIES R G, SNIPPERT H J, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche[J]. Nature, 2009, 459(7244): 262-265. |
| [13] | SATO T, STANGE D E, FERRANTE M, et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium[J]. Gastroenterology, 2011, 141(5): 1762-1772. |
| [14] |
RAHRMANN E P, SHORTHOUSE D, JASSIM A, et al. The NALCN channel regulates metastasis and nonmalignant cell dissemination[J]. Nat Genet, 2022, 54(12): 1827-1838.
doi: 10.1038/s41588-022-01182-0 pmid: 36175792 |
| [15] |
MAO Y N, WANG W, YANG J W, et al. Drug repurposing screening and mechanism analysis based on human colorectal cancer organoids[J]. Protein Cell, 2024, 15(4): 285-304.
doi: 10.1093/procel/pwad038 |
| [16] | KATO H, TATEISHI K, FUJIWARA H, et al. MNX1-HNF1B axis is indispensable for intraductal papillary mucinous neoplasm lineages[J]. Gastroenterology, 2022, 162(4): 1272-1287.e16. |
| [17] | ZHAO H, CHENG Y L, KALRA A, et al. Generation and multiomic profiling of a TP53/CDKN2A double-knockout gastroesophageal junction organoid model[J]. Sci Transl Med, 2022, 14(673): eabq6146. |
| [18] |
DAYTON T L, ALCALA N, MOONEN L, et al. Druggable growth dependencies and tumor evolution analysis in patient-derived organoids of neuroendocrine neoplasms from multiple body sites[J]. Cancer Cell, 2023, 41(12): 2083-2099.e9.
doi: 10.1016/j.ccell.2023.11.007 pmid: 38086335 |
| [19] | GRIGER J, WIDHOLZ S A, JESINGHAUS M, et al. An integrated cellular and molecular model of gastric neuroendocrine cancer evolution highlights therapeutic targets[J]. Cancer Cell, 2023, 41(7): 1327-1344.e10. |
| [20] | LI Z C, XU H B, GONG Y Q, et al. Patient-derived upper tract urothelial carcinoma organoids as a platform for drug screening[J]. Adv Sci, 2022, 9(4): e2103999. |
| [21] |
PONSIOEN B, POST J B, BUISSANT DES AMORIE J R, et al. Quantifying single-cell ERK dynamics in colorectal cancer organoids reveals EGFR as an amplifier of oncogenic MAPK pathway signalling[J]. Nat Cell Biol, 2021, 23(4): 377-390.
doi: 10.1038/s41556-021-00654-5 pmid: 33795873 |
| [22] | HERPERS B, EPPINK B, JAMES M I, et al. Functional patient-derived organoid screenings identify MCLA-158 as a therapeutic EGFR × LGR5 bispecific antibody with efficacy in epithelial tumors[J]. Nat Cancer, 2022, 3(4): 418-436. |
| [23] | ROOVERS R, HERPERS B, JAMES M, et al. Abstract 32: Preclinical evaluation of MCLA-158: a bispecific antibody targeting LGR5 and EGFR using patient-derived colon carcinoma organoids[J]. Cancer Res, 2017, 77(13_Supplement): 32. |
| [24] | COHEN E E, FAYETTE J, DASTE A, et al. Abstract CT012: Clinical activity of MCLA-158 (petosemtamab), an IgG1 bispecific antibody targeting EGFR and LGR5, in advanced head and neck squamous cell cancer (HNSCC)[J]. Cancer Res, 2023, 83(8_Suppl): CT012. |
| [25] | PAUL S M, MYTELKA D S, DUNWIDDIE C T, et al. How to improve R&D productivity: the pharmaceutical industry’s grand challenge[J]. Nat Rev Drug Discov, 2010, 9(3): 203-214. |
| [26] |
ONOZATO D, YAMASHITA M, NAKANISHI A, et al. Generation of intestinal organoids suitable for pharmacokinetic studies from human induced pluripotent stem cells[J]. Drug Metab Dispos, 2018, 46(11): 1572-1580.
doi: 10.1124/dmd.118.080374 pmid: 29615438 |
| [27] |
KATSUDA T, KAWAMATA M, HAGIWARA K, et al. Conversion of terminally committed hepatocytes to culturable bipotent progenitor cells with regenerative capacity[J]. Cell Stem Cell, 2017, 20(1): 41-55.
doi: S1934-5909(16)30347-2 pmid: 27840021 |
| [28] |
VOGES H K, MILLS R J, ELLIOTT D A, et al. Development of a human cardiac organoid injury model reveals innate regenerative potential[J]. Development, 2017, 144(6): 1118-1127.
doi: 10.1242/dev.143966 pmid: 28174241 |
| [29] | TAKASATO M, ER P X, CHIU H S, et al. Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis[J]. Nature, 2016, 536(7615): 238. |
| [30] | 国家药品监督管理局药品审评中心. 人源干细胞产品非临床研究技术指导原则[Z]. 2024. |
| Center for Drug Evalution, National Medical Products Administration. Guidelines for non clinical research techniques of human stem cell products[Z]. 2024. | |
| [31] | ZEHIR A, BENAYED R, SHAH R H, et al. Erratum: mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10 000 patients[J]. Nat Med, 2017, 23(8): 1004. |
| [32] |
YAO Y, XU X Y, YANG L F, et al. Patient-derived organoids predict chemoradiation responses of locally advanced rectal cancer[J]. Cell Stem Cell, 2020, 26(1): 17-26.e6.
doi: S1934-5909(19)30431-X pmid: 31761724 |
| [33] |
NARASIMHAN V, WRIGHT J A, CHURCHILL M, et al. Medium-throughput drug screening of patient-derived organoids from colorectal peritoneal metastases to direct personalized therapy[J]. Clin Cancer Res, 2020, 26(14): 3662-3670.
doi: 10.1158/1078-0432.CCR-20-0073 pmid: 32376656 |
| [34] | OOFT S N, WEEBER F, SCHIPPER L, et al. Prospective experimental treatment of colorectal cancer patients based on organoid drug responses[J]. ESMO Open, 2021, 6(3): 100103. |
| [35] | LI M, IZPISUA BELMONTE J C. Organoids-preclinical models of human disease[J]. N Engl J Med, 2019, 380(6): 569-579. |
| [36] | LESAVAGE B L, SUHAR R A, BROGUIERE N, et al. Next-generation cancer organoids[J]. Nat Mater, 2022, 21(2): 143-159. |
| [37] |
VAN RENTERGHEM A W J, VAN DE HAAR J, VOEST E E. Functional precision oncology using patient-derived assays: bridging genotype and phenotype[J]. Nat Rev Clin Oncol, 2023, 20(5): 305-317.
doi: 10.1038/s41571-023-00745-2 pmid: 36914745 |
| [38] |
WANG H, NING X F, ZHAO F, et al. Human organoids-on-chips for biomedical research and applications[J]. Theranostics, 2024, 14(2): 788-818.
doi: 10.7150/thno.90492 pmid: 38169573 |
| [39] |
KIM M, PANAGIOTAKOPOULOU M, CHEN C, et al. Micro-engineering and nano-engineering approaches to investigate tumour ecosystems[J]. Nat Rev Cancer, 2023, 23(9): 581-599.
doi: 10.1038/s41568-023-00593-3 pmid: 37353679 |
| [40] | LIU H T, GAN Z Q, QIN X Y, et al. Advances in microfluidic technologies in organoid research[J]. Adv Healthc Mater, 2023: e2302686. |
| [41] | TAO T T, DENG P W, WANG Y Q, et al. Microengineered multi-organoid system from hiPSCs to recapitulate human liver-islet axis in normal and type 2 diabetes[J]. Adv Sci, 2022, 9(5): e2103495. |
| [42] | DORNHOF J, KIENINGER J, MURALIDHARAN H, et al. Microfluidic organ-on-chip system for multi-analyte monitoring of metabolites in 3D cell cultures[J]. Lab Chip, 2022, 22(2): 225-239. |
| [43] |
YANG X Y, LI K Y, ZHANG X, et al. Nanofiber membrane supported lung-on-a-chip microdevice for anti-cancer drug testing[J]. Lab Chip, 2018, 18(3): 486-495.
doi: 10.1039/c7lc01224a pmid: 29309077 |
| [44] |
AUNG A, KUMAR V, THEPRUNGSIRIKUL J, et al. An engineered tumor-on-a-chip device with breast cancer-immune cell interactions for assessing T-cell recruitment[J]. Cancer Res, 2020, 80(2): 263-275.
doi: 10.1158/0008-5472.CAN-19-0342 pmid: 31744818 |
| [45] |
PARK D, SON K, HWANG Y, et al. High-throughput microfluidic 3D cytotoxicity assay for cancer immunotherapy (CACI-IMPACT platform)[J]. Front Immunol, 2019, 10: 1133.
doi: 10.3389/fimmu.2019.01133 pmid: 31191524 |
| [46] | TANG X Y, WU S S, WANG D, et al. Human organoids in basic research and clinical applications[J]. Signal Transduct Target Ther, 2022, 7(1): 168. |
| [47] |
CAKIR B, XIANG Y F, TANAKA Y, et al. Engineering of human brain organoids with a functional vascular-like system[J]. Nat Methods, 2019, 16(11): 1169-1175.
doi: 10.1038/s41592-019-0586-5 pmid: 31591580 |
| [48] |
SHIRURE V S, BI Y, CURTIS M B, et al. Tumor-on-a-chip platform to investigate progression and drug sensitivity in cell lines and patient-derived organoids[J]. Lab Chip, 2018, 18(23): 3687-3702.
doi: 10.1039/c8lc00596f pmid: 30393802 |
| [49] | LIU H T, ZHANG X, LIU J Y, et al. Vascularization of engineered organoids[J]. BMEMat, 2023, 1(3): e12031. |
| [50] | RAJASEKAR S, LIN D S Y, ABDUL L, et al. IFlowPlate-a customized 384-well plate for the culture of perfusable vascularized colon organoids[J]. Adv Mater, 2020, 32(46): e2002974. |
| [51] | PUCA L, BAREJA R, PRANDI D, et al. Patient derived organoids to model rare prostate cancer phenotypes[J]. Nat Commun, 2018, 9(1): 2404. |
| [52] | KARKAMPOUNA S, MANNA F L, BENJAK A, et al. Patient-derived xenografts and organoids model therapy response in prostate cancer[J]. Nat Commun, 2021, 12(1): 1117. |
| [53] | LANDON-BRACE N, LI N T, MCGUIGAN A P. Exploring new dimensions of tumor heterogeneity: the application of single cell analysis to organoid-based 3D in vitro models[J]. Adv Healthc Mater, 2023, 12(26): e2300903. |
| [54] | HUANG L X, XU Y Q, WANG N, et al. Next-generation preclinical functional testing models in cancer precision medicine: CTC-derived organoids[J]. Small Methods, 2024, 8(1): e2301009. |
| [55] | KINOSHITA K, TSUKAMOTO Y, HIRASHITA Y, et al. Efficient establishment of bile-derived organoids from biliary cancer patients[J]. Lab Invest, 2023, 103(6): 100105. |
| [56] |
TUVESON D, CLEVERS H. Cancer modeling meets human organoid technology[J]. Science, 2019, 364(6444): 952-955.
doi: 10.1126/science.aaw6985 pmid: 31171691 |
| [57] |
AISENBREY E A, MURPHY W L. Synthetic alternatives to Matrigel[J]. Nat Rev Mater, 2020, 5(7): 539-551.
doi: 10.1038/s41578-020-0199-8 pmid: 32953138 |
| [58] | CLINTON J, MCWILLIAMS-KOEPPEN P. Initiation, expansion, and cryopreservation of human primary tissue-derived normal and diseased organoids in embedded three-dimensional culture[J]. Curr Protoc Cell Biol, 2019, 82(1): e66. |
| [59] |
DAO V, YUKI K, LO Y H, et al. Immune organoids: from tumor modeling to precision oncology[J]. Trends Cancer, 2022, 8(10): 870-880.
doi: 10.1016/j.trecan.2022.06.001 pmid: 35773148 |
| [60] |
VOABIL P, BRUIJN M D, ROELOFSEN L M, et al. An ex vivo tumor fragment platform to dissect response to PD-1 blockade in cancer[J]. Nat Med, 2021, 27(7): 1250-1261.
doi: 10.1038/s41591-021-01398-3 pmid: 34239134 |
| [61] | 国家卫生健康委, 教育部, 科技部, 等. 涉及人的生命科学和医学研究伦理审查办法[Z]. 2023. |
| National Health Commission, Ministry of Education, National Health Commission. et al. Methods for ethical review of human life sciences and medical research[Z]. 2023. | |
| [62] | 类器官药物敏感性检测指导肿瘤精准治疗临床应用专家共识(2022年版)编写专家组. 类器官药物敏感性检测指导肿瘤精准治疗临床应用专家共识(2022年版)[J]. 中国癌症防治杂志, 2022, 14(3): 234-239. |
| Expert Consensus on the Clinical Application of Precision Cancer Treatment Guided by Sensitivity Testing of Organoids (2022 Edition) Writing Expert Group. Expert consensus on clinical application of drug sensitivity test of organ-like drugs to guide accurate treatment of tumors (2022 edition)[J]. Chin J Oncol Prev Treat, 2022, 14(3): 234-239. | |
| [63] | NIIKAWA T, HAYASHI Y, SHEPHERD J, et al. Human brain organoids and consciousness[J]. Neuroethics, 2022, 15(1): 5. |
| [64] | JONGH D D, MASSEY E K, VANGUARD CONSORTIUM, et al. Organoids: a systematic review of ethical issues[J]. Stem Cell Res Ther, 2022, 13(1): 337. |
| [65] | Bold Goals for U.S. Biotechnology and Biomanufacturing[Z]. 2023. |
| [66] | Horizon Europe Work Programme 2023-2024[Z]. 2023. |
| [67] | 科技部基础研究司. “十四五”国家重点研发计划“干细胞研究与器官修复”重点专项2021年度项目申报指南[Z]. 2021. |
| Department of Basic Research, Ministry of Science and Technology of People’s Republic of China. Guidelines for the application of key special projects for stem cell research and organ repair under the 14th Five Year Plan of China in 2021[Z]. 2021. | |
| [68] | 科技部基础研究司. “十四五”国家重点研发计划“干细胞研究与器官修复”重点专项2022年度项目申报指南[Z]. 2022. |
| Department of Basic Research, Ministry of Science and Technology of People’s Republic of China. Guidelines for the application of key special projects for stem cell research and organ repair under the 14th Five Year Plan of China in 2022[Z]. 2022. |
| [1] | LIANG Yingyun, CHEN Jianhua. Application progress of oncolytic virus combined with immunotherapy in the treatment of malignant tumors [J]. China Oncology, 2024, 34(7): 686-694. |
| [2] | CHEN Hong, CAO Zhiyun. Recent progress in the construction and application of patient-derived pancreatic cancer organoid models [J]. China Oncology, 2024, 34(6): 590-597. |
| [3] | WU Hongji, WANG Haixia, WANG Ling, LUO Xiaogang, ZOU Dongling. Application progress and challenges of artificial intelligence in organoid research [J]. China Oncology, 2024, 34(2): 210-219. |
| [4] | CHEN Hongyu, SU Pengyu, LUO Wenzi, PANG Dequan, WANG Feiran. A study of relationship between cardiac exposure dose-volume and cardiovascular autonomic dysfunction in radiotherapy [J]. China Oncology, 2024, 34(11): 1036-1044. |
| [5] | JIANG Yuanyuan, WEI Wenfei, WU Jingya, LI Huawen. Application of organoids in drug screening of gynecological malignant tumors [J]. China Oncology, 2024, 34(11): 1053-1060. |
| [6] | TAN Xiaolang, YAO Sha, WANG Guihua, PENG Luogen. Research on uPAR promoting proliferation, migration, and chemoresistance of pancreatic cancer by inhibiting autophagy via MAPK signaling [J]. China Oncology, 2024, 34(10): 944-956. |
| [7] | YANG Ziyi, GU Bingxin, XU Xiaoping, SONG Shaoli. Comparison of 18F-FDG and 68Ga-FAPI PET/CT in the diagnosis of lung metastasis in different malignant tumors [J]. China Oncology, 2023, 33(9): 829-833. |
| [8] | Expert Committee on Immunotherapy of Chinese Society of Clinical Oncology , Professional Committee on Cancer Biotherapy of Shanghai Anticancer Association . Chinese expert consensus on clinical application of recombinant oncolytic adenovirus in the treatment of malignant tumors [J]. China Oncology, 2023, 33(5): 527-548. |
| [9] | LU Yu, XI Yumeng, HE Xiaoming, YANG Shaokun, ZHANG Jia, WANG Lei, HE Chaoxing, XIANG Bai. Advances in the application of co-culture strategies in organoids [J]. China Oncology, 2023, 33(3): 293-302. |
| [10] | Society of Onco-Endocrinology of Chinese Anti-Cancer Association. Chinese expert consensus on immunotherapy for gynecological malignant tumors (2023 edition) [J]. China Oncology, 2023, 33(10): 954-967. |
| [11] | CHEN Zhujun, DING Zhimin, MA Xiaolu, WANG Yanchun, HU Haoyun, LU Renquan, GUO Lin. Analysis of characteristics of infectious pathogens in malignant tumors combined with bloodstream infection and significance of serum glucose detection [J]. China Oncology, 2023, 33(10): 927-935. |
| [12] | CUI Lingjun, TIAN Chao, CHENG Zixuan, ZHENG Jiabin, SU Fei, TAN Huangying. Advances in preclinical research models for gastroenteropancreatic neuroendocrine neoplasm [J]. China Oncology, 2022, 32(9): 779-785. |
| [13] | The Society of Cancer Multidisciplinary Diagnosis and Treatment, China Anti-Cancer Association, The Society of Cancer Endocrinology, China Anti-Cancer Association. Chinese experts consensus on quality control standards for tumor organoids diagnosis and treatment platform (2022 version) [J]. China Oncology, 2022, 32(7): 657-668. |
| [14] | HU Xichun, HU Zhihuang, WANG Biyun, WANG Jialei, TAO Rong, ZHANG Jian, GUO Weijian, CHEN Jie, LUO Zhiguo, LI Ting, HUANG Mingzhu, QIU Lixin, SANG Youzhou. COVID-19 and systemic anti-cancer therapy [J]. China Oncology, 2022, 32(6): 499-511. |
| [15] | Society of Onco-endocrinology of Chinese Anti-Cancer Association. Expert consensus on metformin adjuvant therapy in malignant tumor patients with diabetes mellitus type 2 (2022 edition) [J]. China Oncology, 2022, 32(11): 1121-1132. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
沪ICP备12009617
Powered by Beijing Magtech Co. Ltd
